Review - Journal of Drug and Alcohol Research ( 2021) Volume 10, Issue 11
A Boon To Poorly Soluble Drugs-Lipid Soluble Systems
Sridevi Gowripattapu1*, D. SathisKumar2 and S.Selvamuthukumar12Department of Pharmacy, Annamalai University, India
Sridevi Gowripattapu, Department of Pharmacy, Aditya Pharmacy College, India, Email: drsmk001@gmail.com
Received: 27-Sep-2021;Accepted Date: Oct 11, 2021; Published: 18-Oct-2021
Abstract
A major challenge faced in the expansion of innovatory medicinal dosage forms is their low water solubility, as a result of which the oral bioavailability is very low. Oral biological availability and solubility can be enhanced through the Lipid Based Drug Delivery System (LBDDS) which plays a crucial role in the expansion of medicinal dosage. Moreover, it is possible to adopt the liquid additives, medicament formulations and have synergistic effects with solid, semi-solid and liquid products. LBDDS drugs are scrutinized in several phases to investigate their bioavailability and dissolution rate, as they are poorly water soluble. This work presents the classification, and mechanisms to improve the solubility, bioavailability, components, characteristics and comparison of LBDD systems for oral use.
Keywords
LBDDS; SEDDS; SMEDDS; SNEDDS; Micro Emulsions; Nano-emulsion
Introduction
Nowadays, most of the recent drugs are hydrophobic in nature and are poorly aqueous soluble and that may cause complications while choosing the correct drug delivery system to attain the best bioavailability of these drugs. Factors like poor water solubility and dissolution rate decrease the absorption rate and bioavailability. No doubt, most of the drugs are administered only orally. The oral bioavailability of these medications mainly depends on many factors associated with aqueous solubility, and permeability through lipid membranes [1-3]. Orally administered medications should possess good solubility, which in turn, has good absorption. If solubility is sensitive within the gastric medium, at this stage solubility (low or high) and permeability through lipid membranes (low or high) issues arise. These medications are being categorized into class II (low solubility or high permeability) or class IV (high solubility or low permeability) as stipulated by BCS (Biopharmaceutical classification system). Various approaches have been developed to enhance solubility that attains the best bioavailability of poorly soluble drugs. Amongst the promising drug delivery approaches are lipid based drug delivery systems (LBDDS) [4,5]. This is confirmed when poorly soluble drugs are co-administered with fat meals. LBDDS has recognized that oral availability improves widely.
LBDDSs are of various types of oily suspensions or simple oil solutions to coarse, moderate, multiple and also dry emulsions. LBDDSs are also more complex self-emulsifying drug delivery systems (SEDDS), Self-micro emulsifying drug delivery systems (SMEDDS) or Self Nano emulsifying drug delivery systems (SNEDDS) [6]. Some of the marketed products developed as SEDDS are Calera® and Ritonavir (ABOTT), Targretin® and Bexarotene (NOVARTIS), Convulex® and Valproic Acid (PHARMACIA), Lipirex ® and Fenofibrate (SANOFI-AVENTIS) [7-10].
The present review focuses mainly on differentiating, oral applications, mechanisms of various lipid based drug delivery systems in enhancing solubility and bioavailability of poor water soluble drugs. Different methods are present to differentiate LBDDSs like SEDDS, SMEDDS and SNEDDS.
Lipid Based Formulations
This lipid based carrier system has been designed to attain local effects, and to regulate and enhance drug delivery of medications [6]. LBDDS contains a mixture of lipids, surfactants and additionally a hydrophilic co-solvent. The Lipid formulation classification systems were categorized into four (I-IV). The LBDDS structure and characteristics are depicted in Table 1 [11].
| Structure Classes | I | II | III a | III b | IV |
|---|---|---|---|---|---|
| Percentage Compositions | |||||
| Hydrophilic surfactants (HLB>12) | 0 | 0 | 0 | 20-50 | 20-80 |
| Lipophilic Surfactants (HLB<12) | 0 | 20-60 | 20-40 | 0 | 0-20 |
| GLYCERIDES (mono, di, triglycerides) | 100 | 40-80 | 40-80 | <20 | 0 |
| Co-solvents | 0 | 0 | 0-40 | 20-50 | 0-80 |
| Characteristics | Non-dispersing, simple oil solution requires lipid digestion. | SEDDS without water soluble components having Self emulsifying ability | SEDDS/SMEDDS With water soluble components having Self emulsifying ability | SMEDDS With water soluble components with low oil content | Type IV contains surfactants,cosolvents and Oil free formulation |
Table 1: Classification of LBDDSs and their properties
Advantages and Disadvantages
Type I is safe and good compatible, Type II has an unlikely solvent capacity on dispersion, Type IIIa forms clear emulsion, which makes drug absorption without digestion, Type IIIb forms Transparent emulsion, which makes drug absorption without digestion, and Type IV has good solvent capacity for drugs. It disperses and forms a micellar solution. Type I has a solvent capacity limited to lipophilic drugs only; Type II formed an emulsion and has particle sizes ranging from 0.25 μm to 0.2 μm. Type IIIa has a solvent capacity lost upon dispersion and is less easily digested. Type IIIb has a solvent capacity partially lost upon dispersion and less easily digested, and Type IV is found to have the risk of drug precipitation upon dispersion and may not be digestible [12,13].
Mechanism
LBDDSs are more advantageous in enhancing oral bioavailability. SNEDDS are more advantageous over SEDDS/SMEDDS because SNEDDS is smaller in particle size, and this increases the particle’s surface area with low free energy. SNEDDS enhances the permeation of poorly water-soluble drugs, oral absorption and oral bioavailability due to the presence of surfactants and co- surfactants. But Stability is the main disadvantage of SNEDDS and it can be overcome by converting Liquid SNEDDS into Solid SNEDDS.
LBDDSs are called SEDDSs. SMEDDSs are formed by mixing water soluble components (surfactants/co-solvents) with the oil part that belongs to category III [11]. SNEDDS are a mixture of oils (lipids/fatty acids) formed by surfactants, co-surfactants, co-solvents and an aqueous phase that belongs to category IV. In SNEDDS, the usage of oil may form a turbid emulsion and this may be avoided by not using turbid oil like lipids/fatty acids [14-16].
Factors Affecting LBDDS
Some of the factors that influence the LBDDS are as follows:
• Mean droplet size: Reducing the droplet size, decreases interfacial tension and results in increasing the surface area of the particle size, thereby providing faster drug release from the emulsion in a controlled and reproducible manner.
• API lipophilicity: The drugs that are (log p>5) i.e., highly hydrophobic and avoid first pass metabolism by portioning into chylomicrons and thus are taken up into the lymphatic system.
• Nature of the lipids: Absorption depends on the nature of the lipids. Long chain triglycerides provide better absorption than medium chain triglycerides in SMEDDS.
• Lipid digestion: The Proper lipid vehicle has to be selected to avoid fluctuations in the absorption rate.
Types of Lipid-Based Drug Delivery Systems
There are different types of LBDDSs like simple drugs in lipid solutions or suspensions, emulsions, SEDDS, SMEDDS and SNEDDS. According to the Lipid-based classification system, SEDDSs are classified as Type II and IIIa systems. SEDDSs are formulated with the mixing of drugs, lipid vesicles and non-ionic surfactants without water that is accepted to exist as isotropic transparent solutions. SEDDS having the property of self-emulsifying immediately forms fine oil in water emulsions by gastrointestinal motion produced by gastrointestinal fluids. SEDDSs are formulated in soft and hard gelatin prepared by using polymers like HPMC (Hydroxypropyl Methyl Cellulose) [16-18].
SMEDDSs produce smaller emulsion droplets upon dilution that is transparent or translucent stable dispersion and have a droplet size <100 nm, whereas SEDDS is distinguished by having a size <300 nm. SMEDDS contains relatively high concentrations of surfactants (30% to 60%, m/m) and also hydrophilic co-solvents like propylene glycol, polyethylene glycols, which form micro emulsion upon dilution with aqueous media and hence, termed as micro emulsion [19-22].
The IIIb systems are known to be SNEDDS that are formed as transparent fine emulsions or dispersions of oil in water spontaneously, having a size of 200 nm upon dilution with water by continuous stirring. According to LBCS, formulations like SNEDDS are called as type IV systems, because SNEDDS contains one /more hydrophilic solvents, co-surfactants/ co-solvents, that are termed as oil free formulations because they formcolloidal micellar dispersions upon dilution in the aqueous phase. SNEDDS are thermodynamically unstable formulations and can be stabilized by converting liquid SNEDDS into solid SNEDDS to maintain stability by formulating dispersions using different techniques [23,24]. The differentiation of LBCS into SEDDS/ SNEDDS/SMEDDS characteristics that are termed as water free systems is described in the following Table 2 [16,25-27].
| Characteristic | SEDDS | SMEDDS | SNEDDS |
|---|---|---|---|
| Composition | Contains drug, surfactant, oil (lipid phase) having the self-emulsifying ability upon exposure to GI fluids. -simple binary formulations | Contains drug, surfactant, co-surfactant, oil phase (lipid phase) and it may also contain hydrophilic co-solvent (optional). | Consists of surfactant/ co-surfactant, oil phase (lipid phase) hydrophilic co-solvent. |
| Lipid droplet size in emulsion | Having 200 nm-5µm/100-300 nm that provide large surface area for absorption and form turbid emulsion. | Having <50/<100nm/<140nm that provide large surface area for absorption and clear or translucent emulsion is formed. | Having <100nm, <200 nm and transparent dispersion is formed. |
| Dispersibilty and Solubilizing capacity | All three systems have high solubilizing capacity and enhanced dispensability | ||
| Dispersions stability | Thermodynamically unstable | Thermodynamically stable | Kinetically stable formulations. |
| Formulation techniques | Ternary phase diagrams is used for the development and optimization of SEDDS | Optimization of SMEDDS is done by pseudo-ternary phase diagrams and order of mixing for pre-selected components is not important. | Order of mixing for pre-selected components is important. |
| Liquid SEDDS/SMEDDS/SNEDDS are formulated in the form of solid dosage forms like capsules, tablets, pellets. | |||
Table 2: Differentiation of LBDDS
Criteria for Selection of Excipients for Lipid-Based Formulations
For developing a LBDDS, the selection of excipients is important to produce an efficacious dosage form. API should blend in suitable oil, surfactant, co-solvents and excipients of the best choice and should possess the following properties [28].
1. Non-toxic, Inert
2. Should provide good self – emulsification.
3. Self-dispensability
4. Digestibility of the suitable excipients
5. Purity and chemical stability of excipients.
Oil phase
While selecting the oil phase for formulating, LBDDS should possess properties like chemical stability, polarity, interfacial tension in the water phase, density, viscosity, and phase behavior [15]. For hydrophobic drugs, it may impact on disintegration and may add lymphatic transport leading to drug precipitation in GIT. The oil phase is made up of glycerides, (mixture of mono, di and triglycerides) consisting of long / medium chain unsaturated fats. Additionally, surfactants like polar oils are also used. The following Table 3 describes the characteristics of the glycerides [29- 31]. According to the review data, it was concluded that MCT was selected for LBDDS due to its better properties like solubilization, self-emulsification capacity and better stability of API than the LCT [32]. Mono and diglycerides have good self-emulsification capacity and better solvency properties due to their amphiphilic nature compared with triglycerides [33].
| Class | Example | Characteristics |
|---|---|---|
| Long chain triglycerides (LCT) | Peanut oil, coconut oil, Castrol oil, sesame oil, sunflower oil etc. | Exhibits GRAS status, easily ingested, digested and are absorbed have poor self- dispersing properties of LCT and lower loading capacity with intermediate log P values. It is having high solubilizing capacity after being dispersed of the formulation. |
| Medium chain triglycerides (MCT) | Captex® 355, caprylic/capric triglycerides, palm seed oil etc. | Have good self-dispersing for less lipophilic drugs. MCT semi-synthetic hydrogenated double bonds are well resistant to oxidation. |
| Mixed, mono, di, triglycerides | Capryol®, Myrj®, Imwitor® 908, 308 etc | Surfactants have better properties due to their amphiphilic nature that is having better self-dispersing ability, high solubilizing capacity for poorly water-soluble drugs. |
Table 3: Characteristics of the Glycerides
Surfactants
The Selection of a suitable surfactant like emulsifier is a very important factor that influences the formation of the LBDDS structure factors like pH, ionic strength and temperature variation are also influenced by the emulsifier being used. The stability of emulsions is decided by the usage of a combination of surfactants (emulsifier)/co-surfactants(co-emulsifier) with their respective HLB values. An emulsifying agent is used to maintain the steady state between the internal and external phases of the oil in water emulsion interphase by decreasing the interfacial tension and preventing conglomeration. The differentiation of hydrophilic or lipophilic surfactants is described according to the HLB values and displayed in Table 4 [34].
| HLB VALUE | Type of the surfactant |
| Low HLB (<10) | Unsaturated polyglycolized glycerides (macro-golglycerides), Labrafil® M1944 CS, M2125CS. Sorbitan esters Campul®, Campul® S, Span® 20 and 40. Mixtures containing propylene glycol/ MCT, ethanol, mixtures of phosphatidylcholine and phosphatidylcholine. Poly-ethoxylated alkyl ethers like Brijs®. |
| Low HLB (>10) | Poly-sorbates like Tween® 20,40,60,80. Poly-ethoxylated fatty acid ethers like Myrj® 52, Solutol®, HS15. Poly-ethoxylated glycerides like Labrasol®. Polyoxyl 35 castor oil derivatives like Cremophor® EL. Polyoxyl 40 hydrogenated castor oil derivatives like Cremophor® RH40 Poloxamer 188, 407 are belonging to polyoxyethylene polyoxypropylene block copolymer. Saturated polyglycolized glycerides Lauroyl macrogolglycerides like Gelucire® 44/14 Stearoyl macrogolglycerides like Gelucire® 50/13. |
Table 4: Differentiation of surfactants
The selection of suitable surfactants is a critical step during formulations to maintain the safety of the dosage form. Non-ionic surfactants like Poly-ethoxylated lipid derivatives are mostly preferred and used [35,36]. Poly-ethoxylated lipid derivatives consist of fats, alcohols/glycerides that are linked with repeated polyethylene oxide units by ester linkage (glycerides, unsaturated fats) and by ether linkage (alcohols). Hydrophilic attributes are given to polyethylene groups [19,35]. Natural origin surfactants are more preferred than synthetic surfactants because natural surfactants had more safety over synthetic ones [37]. Non-ionic surfactants had reduced toxic effects over cationic and ionic surfactants and also offer better adjustment of pH and ionic strength. The stability of SMEDDS is attained by using surfactants ranging from 30% to 60% [34,38].
Co-Surfactants/co-solvents
During the formulation of LBDDS, co-solvents are used to improve the limitation of the solubilization of substituted drugs and also enhance the dispensability of hydrophilic surface active agents in the oil phase. Medium chain alcohols having 8 to 12 C atoms produce better results; otherwise, can include ethylene glycol, propylene glycol, and glycerol to produce a satisfactory dosage form [11,34,36]. Selection of co-solvents in the case of hydrophobic drugs is taken into consideration to avoid precipitation of hydrophobic drugs after dilution of co-solvents’ formulation in the aqueous phase. Complexation of LBDDS is enhanced by the usage of co-solvents. LBDDSs are stable in the absence of solvents like alcohols/ volatile solvents [6,32].
Oral bioavailability enhancement techniques for poorly water soluble medications
The LBDDS self-emulsifying systems offer better bioavailability by providing continuous supply of medication and improving drug absorption. The drug is protected from enzymatic or synthetic degradation by incorporating medication into the lipid beads [6,38]. Different techniques are used for enhancing oral bioavailability by increasing solubilization, extension of medication retention in the stomach, modifying bio-chemical barriers and physical barriers during absorption of medications and through stimulating intestinal lymphatic transport [12,16,28,39].
Discussion
During the formulation of LBDDS, type III and IV have the nature of drug precipitation [28,37,38]; if the surfactants/ co-surfactants/co-solvents are not properly selected this may affect the bioavailability of medications. To overcome this problem, while selecting excipients the following parameters have to be taken into consideration, like lipid digestion, mean droplet size of emulsion, Active pharmaceutical drug lipophilicity, and compatibility or attractiveness of lipids [40-43]. By using SEDDS, SMEDDS and SNEDDS, scientists have improved the bioavailability of different poorly soluble drugs. The drug details are mentioned in Tables 5-7 (Figures 1 and 2).
| Drug name | Excipients used | Increased fold of oral bioavailability | Reference |
|---|---|---|---|
| Paclitaxel | HPMC on SEDDS | 22.6% | Ping Gao et al., (2003) [44] |
| Itraconazole | Transcutol\ pluronic\L64 and tocopherol acetate | 2.8-fold higher | Chong – Kook kim et al., (2006) [45] |
| Dexibuprofen | Labrasol, Capryol90, Labrafil M 1944 CS | 2-fold higher | Prabagar Balakrishnan et al., (2009) [46] |
| PNU- 91325 | 30% W/W Cremophor, 9% PEG 400, 5% DMA, 18% Pluronic L44.\, 20% HPMC | 76% higher | Ping Gao et al., (2004) [47] |
| Docetaxel | Capryol 90, Vitamin E TPGS, Gelucire 44/14 and Transcutol HP (ratio of 32.7/29.4/8.3/29.6 ) | 3.19-fold higher | Guru R. Valicherla et al., (2016) [48] |
| Nevirapine | 32.5% oleic acid, 44.16% Tween 20, and 11.9% polyethylene glycol 600 as oil | Increased bioavailability | Ram Prasad et al., (2015) [49] |
| Atorvastatin Calcium |
Captex 355, Captex 355 EP/NF, Ethyl Capmul MCM, CapmulPG-8, Gelucire 44/14, Tween 80, Tween 20, and PEG 400 oleate | Increased bioavailability | Pawan J. et al., (2011) [50] |
| Silybin | Labrafac CC, Cremophor RH40, Labrasol, and 5% HPMC | S- SEDDS Increased bioavailability than SEDDS | Y. Wei et al., (2012)5 [51] |
| Cyclosporine | NC-(25/125) and NC-(50/125) | NC-SEDDS Increased bioavailability | M.-J. Park et al., (2013) [52] |
| Vancomycin | (25% Capmul 808G EP/NF, 37.5% Cremophor RH 40, 37.5%), (26.5%Capmul 808G EP/NF, 33.2% Cremophor RH 40, 13.8% Transcutol, 26.5% DMSO) and F3 (28.8% Captex 8000,35% Cremophor EL, 20% Transcutol, 16.2% DMSO) | 219-fold improved permeation through intestinal mucosa and Increased bioavailability | S. Zaichik et al., (2019) [53] |
| Cannabidiol (CBD) | Dilution of Hemp extracts MCT and oil mixture. | Cmax4.4 fold higher and a 2.85-/1.70-foldhigher AUC0-8h/0-24h |
Katharina Knaub et al., (2019) [54] |
| Ibuprofen | 24.10 mg Capmul PG8 and 71.02 mg Cremophor EL | Increases drug release 74.24% | Sadika Akhter et al., (2012) [55] |
Table 5: List of drugs whose bioavailability is enhanced by SEDDS
| Drug name | Excipients used | Increased fold of oral bioavailability | Reference |
|---|---|---|---|
| Acyclovir | Tween 60 (60%), glycerol (30%) and sunflower oil (9%) | 3.5-fold increased | D. Patel et al[56] |
| Vinpocetine | Labrafac: oleicacid :CremophorEL:Transcutol P_40 : 10 : 40 : 10 (w/w) | (-)VIP-SMEDDS and (+) VIP-SMEDDS was 1.85- and 1.91-fold increased. | Ying CHEN et al [57] |
| Berberine Hydrochloride | 40% (w/w) of ethyl linoleate and oleic acid (2:1), 35% (w/w) Tween-80 and 25% (w/w) glycerol | 2.42-fold increased | Jia-Xiao Zhu et al [58] |
| [6]-Gingerol | (ethyl oleate), (Cremophor EL35) and (1,2-propanediol) | 6.58-fold increased | Yang Xu et al [59] |
| Leuprorelin | (30% (m/m)Cremophor EL, 30% (m/m) Capmul MCM, 10% (m/m) propylene glycol and 30% (m/m) Captex 355) | 17.2-fold increased | Fabian Hintzen et al [60] |
| Atorvastatin | Labrafil, propylene glycolandCremophor RH40 | 1.5 times increased | HaiRong Shen et al [61] |
| Methotrexate | Castor oil,Tween® 80, and Plurol® (27:63:10), solid carrier (calciumsilicate) | 2.04- and 3.41-fold increments in AUC and Cmax increased bioavailability | Kim et al [62] |
| Pueraria lobata Isoflavone | (ethyl oleate), (Tween 80), and (Transcutol P) | 2.5-fold increased bioavailability | S. Cui et al [63] |
| Cathepsin K inhibitor (HL235) | 5.0% Capmul MCM EP , 75.0% Tween 20 and20.0% Carbitol | 3.22-fold increased bioavailability | Voradanu Visetvichaporn et al [64] |
| Felodipine | Miglyol-812,Cremophor-RH 40, Tween 80 and Transcutol- P | 2-fold greater bioavailability | B. Jing et al [65] |
| Pueraria Flavones | PF, Crodamol GTCC, Maisine 35-1, Cremophor RH 40, 1,2-propyleneglycol and polyethylene glycol 6000 (PEG6000) | PF-SMEDDS dropping pills increase 1.69- and 2.36- fold | Qingxiang Guan et al [66] |
| Vardenafil HCl trihydrate (VDN) | Cremophor EL and Tween 20 | Tween 20 VDN SMEDDS showed 2.8-fold increase in bioavailability | Kinjal J Parikh et al [67] |
| Ferulic Acid (FA) | Glyceryltriacetate, a mixture of OP-10 and labrasol (2:1, v/v), andPEG 400 (0.2: 0.45: 0.3) | 185.96% improved Bioavailability | Chang-Shun Liu et al [68] |
| Curcumin | 57.5% OP: Cremophor EL = 1:1), 30.0% (PEG 400) and 12.5% (ethyl oleate) | Increased Bioavailability | J. Cui et al [69] |
| 20(S)-Protopanaxadiol | 20(S)-PPDwith carboxymethyl cellulose (CMC). | 3 – fold increased | Bing Wang et al [70] |
| Candesartan Cilexetil | C7IIB formulation | Enhanced solubility and dissolution of candesartan cilexetil. | Shukla et al [71] |
| Rapamycin | PEG 400/ethanol,glycerol/ethanol, propylene glycol, glycerol formal, Transcutol P | Improved solubility, dissolution and bioavailability | M. Sun et al [72] |
| Doxorubicin Hydrochloride | Tocopheryl-Polyethylene Glycol-1000-Succinate (TPGS) and Capmul | 420% enhancement of oral bioavailability | Derajram M et al [73] |
| Nintedanib | MCT, RH 40and ethylene glycol | Improved permeability 3.0-fold in the entire intestine and 3.2-fold in the colon and increases bioavailability | Liu et al[74] |
| Curcumin | Ethyloleate, Tween 80, and Transcutol® P | 29-fold increased bioavailability | Umesh Kannamangalam Vijayan et al [75] |
| Iloperidone | Capmul MCM, Labrafac WL 1349, Lauroglycol 90 and PEG 600 and Syloid XDP and Aerosil 200 15:1 w/w for liquisolid compacts | 3.80 fold and 2.19 fold improved in oral bioavailability of solid SMEDDS and liquisolid compacts | Dinesh Suram et al [76] |
Table 6: List of drugs whose bioavailability is enhanced by SMEDDS
| Drug name | Excipients used | Increased fold of oral bioavailability | Reference |
|---|---|---|---|
| Darunavir | Capmul MCM C8 , Tween 80 and Transcutol P | oral bioavailability improved | Spandana Inugala et al [77] |
| Quercetin | castor oil, Tween 80, Cremophor RH 40, and PEG 400 (20:16:34:30, w/w) | oral bioavailability improved | Tran et al [78] |
| Flurbiprofen | Labrafil M 1944 CS/Labrasol/Transcutol HP (12.5/80/7.5%) | oral bioavailability improved | Jun Hyeok Kang et al [79] |
| Lacidipine (LCDP) | A mixture of Labrafil®/Capmul®), (a mixture of Cremophor®/Tween® 80) and the co-surfactant. | Oralabsorption improved | Emad B et al [80] |
| Efavirenz | Cr EL and Brij 35 in ratio of 1:1. | Oral bioavailability improved | Prakash C et al [81] |
| Triple antioxidants QT (flavonol), RES (stilbenoid) and GEN (isoflavone phytoestrogen) | Cremophor-RH40 and Tween20 | Enhanced Oral bioavailability | Shailja Tripathi et al [82] |
| Chlorpromazine | small chain triglycerides (SCT), medium chain triglycerides (MCT), and long-chain triglycerides (LCT) | 6-fold improved oral bioavailability | Jeand Baloch et al [83] |
| Dabigatran etexilate | Oils (25–45%), surfactant (30–50%) and co-surfactant (ranged from 0.5:1 to 8:1) | 531.80% relative bioavailability and enhanced oral bioavailability | Fujuan Chai et al [84] |
| Celecoxib | capryol 90, cremophor RH 40 and propylene glycol | improved oral bioavailability | S.L. Harikumar et al. [85] |
| Carvedilol | Capmul PG8, Cremophor EL and Transcutol HP | several fold improved in the permeability and absorption potential | Bhupinder Singh et al. [86] |
Table 7: List of drugs whose bioavailability is enhanced by SMEDDS
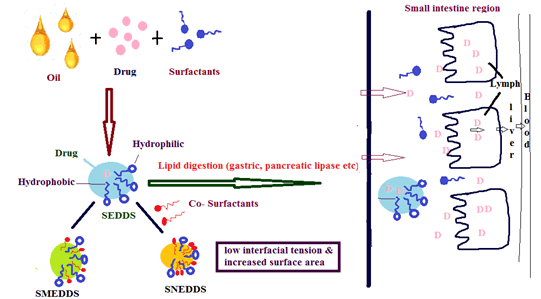
Figure 1: Mechanism of LBDDS
Figure 2: Graphical abstract
Conclusion
A Lipid Based Drug Delivery System (LBDDS) has been proved to be a promising approach to enhance the absorption, and oral bioavailability of poorly water soluble drugs by spontaneous emulsification and formation of emulsion, micro emulsion and nano emulsion. In the development of LBDDS, different surfactants, co-surfactants are used which may cause compatibility, and stability issues of LBDDS. These issues can be overcome by converting liquid LBDDS (SEDDS/SMEDDS/SNEDDS) into solid LBDDS.
References
- A. J. Humberstone, W. N. Charman, Lipid based vehicles for the oral delivery of poorly water soluble drugs, Advanced drug delivery reviews, 25 (1997), 103-128.
- B. Bittner, R. J. Mountfield, Intravenous administration of poorly soluble new drug entities in early drug discovery: The potential impact of formulation on pharmacokinetic parameters, Current opinion in drug discovery and development, 5 (2002), 59-71.
- U. Chaudhary, N. Nagaich, V. K. Gulati, R. L. Sharma Khosa, Enhancement of solubilization and bioavailability of poorly soluble drugs by physical and chemical modifications: A recent review, J Adv Pharm Educ Res, 2 (2012), 32–67.
- T. Loftsson, M. E. Brewster, M. Masson, Role of cyclodextrins in improving oral drug delivery, Am J Adv Drug Deliv, 2 (2004), 261-275.
- J. Ye, H. Wu, C. Huang, W. Lin, C. Zhang, B. Huang, X. Long, Comparisons of in vitro Fick’s first law, lipolysis, and in vivo rat models for oral absorption on BCSII drugs in SNEDDS, Int J Nanomedicine. 14 (2019), 5623.
- B. K. Nanjwade, D. J. Patel, R. A. Udhani, F. V. Manvi, Function of lipids for enhancement of oral bioavailability of poorly water soluble drugs, Sci. Pharm. 79 (2011), 705–727.
- K. Kohli, S. Chopra, D. Dhar, S. Arora, R. K. Khar, Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability, Drug Discov Today, 15 (2010), 958–965.
- S. B. Murdandea, M. J. Gumkowskia, Development of a self-emulsifying formulation that reduces the food effect for torcetrapib: An overview, Int J Pharm. 51 (2008), 15–22.
- J. Parul, A. Geeta, K. Amanpreet, Bioavailability enhancement of poorly soluble drugs by SMEDDS: A review, J. Drug Deliv. Ther. 3 (2013), 98–109.
- S. Kalepu, M. Manthina, V. Padavala, Oral lipid-based drug delivery systems: An overview, Acta Pharm Sin B 3 (2013), 361-372.
- S. Saroy, D. A. Baby, M. Sabitha, Current trends in lipid based delivery systems and its applications in drug delivery, Asian J Pharm Clin Res, 5 (2012), 4–9.
- H. J. C. Porter, L. N. Trevaskis, W. N. Charman, Lipids and lipid based formulations: Optimizing the oral delivery of lipophilic drugs, Nat Rev Drug Discov, 6 (2007) 231–248.
- W. C. Pouton, J. H. C. Porter, Formulation of lipid based delivery systems for oral administration: Materials, methods and strategies, Adv Drug Deliver Rev, 60 (2008), 625–637.
- R. Nagaraju, D. Saritha, P. S. C Bose, G. Ravi, V. Ravi, A review on current status of self-emulsifying drug delivery systems, Int Res J Med Bio Sci. 3 (2019).
- G. Radha, K. T. Sastri, S. Burada, J. Rajkumar, A systematic review on self-micro emulsifying drug delivery systems: A potential strategy for drugs with poor oral bioavailability, Int J App Pharm, 11 (2019), 23-33.
- B. V. Rajesh, T. K. Reddy, G. Srikanth, V. Mallikarjun, P. Nivethithai, Lipid based self-emulsifying drug delivery system (SEDDS) for poorly water soluble drugs: A review, J Glob Pharma Technol, 2 (2010), 47–55.
- S. Charman, Self-emulsifying drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compound, Pharm Res, 9 (1992), 87–93.
- R. Neslihan Gursoy, S. Benita, Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs, Biomed, Pharmacother, 58 (2004) 173–182.
- J. B. Cannon, Strategies to formulate lipid based drug delivery systems, Am Pharm Rev, 14 (2011), 84.
- T. G. Mason, J. N. Wilking, K. Meleson, C. B. Chang, S. M. Graves, Nanoemulsions: Formation, structure and physical properties, J Phys Condens. Mat, 18 (2006) 635–666.
- H. D. Oh, H. J. Kang, W. D. Kim, J. B. Lee, O. J. Kim, et al., Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier, Int J Pharm, 420 (2011) 412–418.
- K. B. Kang, J. S. Lee, S. K. Chon, Development of self-micro emulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs, Int J Pharm, 274 (2004), 65–73.
- S. Tenjarla, Microemulsions: An overview and pharmaceutical applications, Crit. Rev. Ther. Drug, 16 (1999), 461–521.
- R. Santhosh kumar, R. Sureshkumar, A review on solid supersaturable snedds. Research, J pharm and tech, 13 (2020), 3530-3535.
- B. Singh, S. Bandopadhyay, R. Kapil, R. Singh, O. Katare, Self-emulsifying drug delivery systems (SEDDS): Formulation development, characterization, and applications, Crit Rev Ther Drug, 26 (2009) 427–521.
- P. K. Suresh, S. Sharma, Formulation and in vitro characterization of self-nanoemulsifying drug delivery system of cinnarizine, Int J Compr Pharm, (2011) ISSN 0976-8157.
- D. J. McClements, J. Rao, Food grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate and potential toxicity, Crit Rev Food Sci, 51 (2011), 285–330.
- K. Mohsin, A. A. Shahba, F. K. Alanazi, Lipid based self-emulsifying formulations for poorly water soluble drugs an excellent opportunity, Ind J Pharm Edu Res, 46 (2012), 88–96.
- M. Stuchlik, Lipid based vehicle for oral drug delivery, Biomed Pap, 145 (2001), 17- 26.
- P. Constantinides, Lipid micro emulsions for improving drug dissolution and oral absorption: Physical and biopharmaceutical aspects, Pharm Res 12 (1995), 1561–1572.
- P. P. Constantinides, J. P. Scalart, Formulation and physical characterization of water in oil microemulsions containing long versus medium chain glycerides, Int J Pharm, 158 (1997) 57– 68.
- M. Grovea, A. Mullertzb, Bioavailability of seocalcitol II: development and characterisation of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides, Eur J Pharm Sci, 28 (2006), 233–234.
- H. N. Prajapati, M. D. Dalrymple, T. M. A. Serajuddin, A comparative evaluation of mono, di and triglyceride of medium chain fatty acids by lipid/surfactant/water phase diagram, solubility determination and dispersion testing for application in pharmaceutical dosage form development, Pharm Res, 9 (2012), 285–305.
- W. C. Pouton, Properties and uses of common formulation lipids, surfactants and cosolvents, in AAPS, Workshop, March (2007).
- Pharmaceutical Excipients, Product Reference Quick Guide, Gattefosse, Version VIII, April (2012).
- N. H. Shah, M. T. Carvajal, C. I. Patel, M. H. Infeld, A. W. Malick, Self-emulsifying drug delivery systems (SEDDS) with polyglycolized glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs, Int J Pharm, 106 (1994) 15–23.
- M. Kimura, M. Shizuki, K. Miyoshi, T. Sakai, H. Hidaka, H. Takamura, T. Matoba, Relationship between the molecular structures and emulsification properties of edible oils, Biosci Bio-tech Bioch 58 (1994), 1258–1261.
- A. Zvonar, Self (micro) emulsifying systems – alternative approach for improving bioavailability of lipophilic drugs, Farm Vestn, 59 (2008), 263–268.
- R. N. Gupta, R. Gupta, R. G. Singh, Enhancement of oral bioavailability of lipophilic drugs from self-microemulsifying drug delivery systems (SMEDDS), Int J Drug Dev Res, 1(2009), 10– 18.
- M. J. Groves, D. A. Degalindez, The self-emulsifying action of mixed surfactants in oil, Acta Pharm Suec, 13 (1976), 361–372.
- S. M. Khoo, A. J. Humberstone, C. J. H. Porter, G. A. Edwards, W. N. Charman, Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine, Int J Pharm, 167 (1998) 155–164.
- K. J. Palinand C. G. Wilson, The effect of different oils on the absorption of probucol in the rat, J Pharm Pharmacol, 36 (1984) 641–643.
- J. H. Lin, W. Chen, J. King, The effect of dosage form on oral absorption of L-365, 260, a po tent CCK receptor antagonist in dogs, Pharm Res, (1991) 8272.

