Research Article: Journal of Drug and Alcohol Research (2021) Volume 10, Issue 3
A comparative Study of Long Acting Injectable Antipsychotic Agents and Oral Agents during COVID-19 Pandemic: Quality of Life, Adherence and Shortage of Supply
Muamar MA Shaheen*, Sara Al Saghair, Hamzah Obeido, Basema Al Kurd, Ibrahim Al Zaro and Manar Al JunaidiMuamar MA Shaheen, Department of Pharmacy and Medical Sciences, clinical pharmacy and practice, Israel, Email: muamarsh@hebron.edu
Received: 28-Dec-2020;Accepted Date: Jan 18, 2021; Published: 26-Feb-2021
Abstract
Background: Long-acting injectable antipsychotics improved markedly patient adherence to psychotropic agents during the past decade. They were used mainly for long-term treatment of schizophrenia. However their role in short term or intermittent use or their effect on quality of life was not elucidated clearly.
Objectives: To assess the impact of Long Acting Antipsychotic agents on quality of life of schizophrenic patients.
Methods: This is a retrospective cohort study of psychiatric patients who were taking LAIs and/or oral antipsychotic drugs at Mohammad Said Kamal Hospital for Mental Illness in Bethlehem and Mental Health Clinic of The Ministry of Health in Hebron city during the period of September 2019 to March 2020.
Results: Fifty one patients were included in this study, 74% males, age 50.69 ± 11.14 years old. Average duration of psychiatric disease was 17.78 ± 11.4 years. It was found that 9.6% patients were on oral dosage form (category I), 80.4% were on LAI and oral antipsychotics (category II), and 10% were on LAIs (Category III). Chi square test showed a significant difference between the 3-categories and GAF score (functionality), p=0.003. However, there was insignificant difference between the three categories and CGI-S(severity of symptoms) scores, p=0.170. When it comes to side effects, there was a significant difference among the three categories and DIEPSS, p=0.049. Kruskal–Wallis Test showed a significant difference between patients in the three categories and number of all drugs, p=0.007. There was also a significant difference between CGI-S-normal group and CGI-S-severe symptoms group and overall number of drugs used, p=0.02. Mann-Whitney test showed a significant difference between number of all drugs used and the use of trihexphenidyl, p=0.001. Also there was a significant difference between number of antipsychotic drugs alone and thrihexphenidyl use, p=0.001. Patients were prescribed LAIs for the following reasons: non-adherence (47%), no reason at all (27.4%), patient dissatisfaction (13.7%), adherence and patient dissatisfaction (5.8%), side
effects, convenience (ease of use), and availability of drug, (1.9%), for each.
Conclusion: Improvement in functionality of schizophrenic patients goes along with use of LAIs either alone or in combination. LAIs improved adherence and minimizes polypharmacy.
Keywords
LAIs; Conventional antipsychotic therapy;Adherence; GAF score; Quality of life; Psychosocial improvement
Introduction
Long-acting antipsychotic injections (LAIs) were introduced in 1966 in an attempt to improve long-term treatment for schizophrenia. Scientists have previously been involved in developing guidelines for the use of injectable antipsychotics [1]. Since then, LAIs had evolved markedly as potential agents of choice for many reasons such as their potential use at the first episode of psychiatric illness, or disease early stage, in addition to their in refractive disorders. Many reviews of guidelines for their use in schizophrenia have evolved over the past decade.
So, why we do need the long-acting (long-term) treatment for schizophrenia and other psychotic disorders in the first place?
A plethora of studies was conducted over the past several decades on LAIs. The debate about indications and contraindications to the use of antipsychotics in general has diminished. It was accepted in a wide range that antipsychotics are indicated for treatment of schizophrenia in both the short term and long term without focusing on subtype of condition, age of patient, or type of appearance. However, there’s less consensus about the suitable duration of treatment, especially for people who have experienced just one episode of the disease.
It was not until the 1980’s that placebo-controlled trials were first conducted for maintenance after one episode [2,3] These studies were generally relatively short-lived (1-2 years), given the potential and predictable duration of schizophrenia [4].
Although these trials, along with the more natural evaluation of relapse prevention [5] support the superiority of persistent antipsychotic drugs compared to placebo or no treatment, relapse rates even in placebo were generally lower than those observed under placebo after multiple seizures of the disease. This observation suggested that a large subgroup of people with first episode disorder is not at risk of relapse when medications are stopped for a year or more. This poses a question over the relative necessity or benefit-to-risk ratio of long-term treatment after one episode. Data from long-term follow-up studies also confirmed the potential heterogeneity of outcomes in schizophrenia, with or without medication [6,7].
It is important to realize that long-term exposure to medicines is not without risks generally. Concerns about the development of delayed dyskinesia [8] and the negative effects of endocrine and metabolism [9] also influenced the benefit-to-risk ratio of longterm treatments. There is another complication in looking at the indications of long-term drug therapy in managing schizophrenia. This includes the nature of the disease itself and its potential impact on cognitive performance, insight, a tendency to suicide or violent behavior and misuse of accompanying materials, and social and professional performance.
A disease that can be associated with exacerbations with loss of insight and decreased interest in taking the drug, as well as a potential risk to itself and others, is a somewhat different set of challenges from those associated with less complex changes in health. Basically, schizophrenia is a complex condition and most patients need long-term treatment because relapse is associated with significant personal costs.
When it comes to antipsychotics, adherence is not just a term of compliance or a measure of taking or not taking the medication. It reflects a deterioration of patient`s mental status, worsening of social performance and loss of marginal functionality which is already compromised by many aspects of the progressive nature of mental condition and side effects of treatment. Non adherence, if not caught early, it will hinder psychosocial improvement and blurred the fine line between clinical improvement and psychosocial perfection amongst a wide range of other risk factors.
There is scarcity of studies that address quality of life of schizophrenic patients and their social integration as an optimal goal of treatment rather than the opponent inevitable destiny of overmedication and/or social isolation.
In this study we are trying to quantify the pain and suffering of schizophrenic patients in between shortage of drug supply and overmedication. In other words, we highlight the need for ongoing drug supply when they are mostly needed and holding unnecessary drugs.
By using global scales for severity of symptoms, functionality assays and side effect evaluation, we clearly define the line between optimal medical treatment that reinforce social performance and over medication or suboptimal therapy that lead to deterioration of patient condition or relapse of psychiatric attack.
Methodology
This is a retrospective cohort study by design where we reviewed complete profiles of all patients who visited the two hospitals mentioned above during the past 6 months. Out patients, 18 years of age or older who were diagnosed with schizophrenia, bipolar depression, and/or other mood and manic conditions who were taking LAIs and/or other antipsychotic or non-psychotropic agents were included in this study.
Clinical Global Impressions-Severity of Illness Scale (CGI-S), Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS), and Global Assessment of Functioning (GAF) were used to determine symptom severity, drug-induced movement disorders, and effect of symptoms on a person`s functionality, respectively. Patients were categorized into 3-therapeutic categories according to dosage form(s) of psychotropic drugs used during the past 6 months; Category I: oral antipsychotics, Category II: combination antipsychotics (oral and LAIs), Category III: LAIs only. A special form was designed to collect data about severity of illness, side effects of psychotropic agents, functionality and quality of life, psychosocial and socio-demographic data from patient’s profiles and from the attending physicians. SPSS version 22 was used to analyze the results.
Results
Fifty one patients who were diagnosed with schizophrenia were included in this study (74% males), age 50.69 ± 11.14 30-89 years old. Table 1 below shows their scociodemographic characteristics, severity of their symptoms, drug-induced movement disorders, and their day-to-day life activities (functionality) represented by scores of CGI-S, DIEPSS, and GAF, respectively.
| Variable | Number (n=51) | Frequency (%) |
|---|---|---|
| Gender | ||
| Male | 38 | 74.5 |
| Female | 13 | 52.5 |
| Education | ||
| University Graduate | 2 | 3.9 |
| High School | 20 | 39.3 |
| Primary school | 18 | 35.3 |
| No education | 11 | 21.5 |
| Age | ||
| 29-48 | 21 | 41.1 |
| 49-68 | 27 | 52.9 |
| = 69 | 3 | 5.8 |
| Family history of psychiatric Illness | ||
| Yes | 9 | 17.7 |
| No | 42 | 82.3 |
| *GAF score | ||
| Nov-20 | 1 | 1.9 |
| 21-30 | 5 | 8.8 |
| 31-40 | 4 | 7.8 |
| 41-50 | 1 | 1.9 |
| 51-60 | 3 | 5.9 |
| 61-70 | 4 | 7.8 |
| 71-80 | 19 | 37.3 |
| 81-90 | 10 | 19.6 |
| 91-100 | 4 | 7.8 |
| Total | 51 | 100 |
| *DIEPSS | ||
| 0 | 1 | 1.9 |
| 2 | 49 | 94.2 |
| 4 | 2 | 3.8 |
| Total | 51 | 100 |
| *CGI-S | ||
| 1 | 7 | 13.7 |
| 3 | 10 | 19.6 |
| 4 | 7 | 13.7 |
| 5 | 5 | 9.8 |
| 6 | 22 | 43.1 |
| Total | 51 | 100 |
*GAF: Global Assessment of Functioning. DIEPSS: Drug-Induced Extrapyramidal Symptoms Scale, and CGI-S: Clinical Global Impressions-Severity of Illness Scale.
Patients were categorized into three categories according to type of dosage form of antipsychotic agents they were on; category I: oral antipsychotic agents (conventional), category II: combination of oral antipsychotics and LAIs, and category III: LAIs only.
The distribution of patients over the three different mental illness scales according to their scores within their categories was established in Table 2. A comparison between the three categories according to dosage form was done using Chi square test. There was a significant difference in GAF score between the three categories, p=0.003, where patients with high scores on GAF were either on combination therapy or IM therapy. There was insignificant difference between the three categories and CGI-S scores, p=0.170.
| Variables | Test statistic | Sig. | |||
|---|---|---|---|---|---|
| GAF score | Oral | Combination* | IM¥ | ||
| 1 – 10 | 0 (0) | 0 (0) | 0 (0) | 26.857 | 0.003 |
| 11 – 20 | 0 (0) | 2.3 (1) | 0 (0) | ||
| 21 – 30 | 0 (0) | 11.4 (5) | 0 (0) | ||
| 31 – 40 | 50 (2) | 4.5 (2) | 0 (0) | ||
| 41 – 50 | 25 (1) | 0 (0) | 0 (0) | ||
| 51 – 60 | 0 (0) | 6.8 (3) | 0 (0) | ||
| 61 – 70 | 25 (1) | 6.8 (3) | 0 (0) | ||
| 71 – 80 | 0 (0) | 40.9 (18) | 33.3 (1) | ||
| 81 – 90 | 0 (0) | 22.7 (10) | 0 (0) | ||
| 91 – 100 | 0 (0) | 4.5 (2) | 66.7 (2) | ||
| Total | 100 (4) | 100 (44) | 100 (3) | ||
| CGI score | |||||
| 1 | 0 (0) | 11.4 (5) | 66.7 (2) | 9.032 | 0.17 |
| 2 | 0 (0) | 0 (0) | 0 (0) | ||
| 3 | 25 (1) | 20.5 (9) | 0 (0) | ||
| 4 | 0 (0) | 13.6 (6) | 33.3 (1) | ||
| 5 | 25 (1) | 9.1 (4) | 0 (0) | ||
| 6 | 50 (2) | 45.5 (20) | 0 (0) | ||
| Total | 100 (4) | 100 (44) | 100 (3) | ||
| DIEPSS score | |||||
| 1 | 0 (0) | 0 (0) | 33.3 (1) | 11.983 | 0.049 |
| 2 | 0 (0) | 0 (0) | 0 (0) | ||
| 3 | 75 (3) | 97.7 (43) | 66.7 (2) | ||
| 4 | 0 (0) | 0 (0) | 0 (0) | ||
| 5 | 25 (1) | 2.3 (1) | 0 (0) | ||
| Total | 100 (4) | 100 (44) | 100 (3) | ||
| Note: The value represents percentage (count) *Combination dosage forms: IM and oral antipsychotic drugs, ÃÂ?Â¥ IM: Intramuscular Injections ÃÂ?§Psychotropic drugs used: 1st gen.: chlorpromazine, thioridazine, respiridone, haloperidol, haloperidol IM, fluphenazine (IM); 2nd gen.: clozapine, quetiapine, olanzapine. | |||||
Table 2: Comparison between the three categories of patients according to dosage form of §psychotropic drugs used and GAF, DEIPPS, or CGI scores.
There was a significant difference among the three categories in regard to DIEPSS, p=0.049. In a sense most patients on combination therapy or IM alone have suffered less side effects comparing to patients on oral dosage form Table 2.
In addition to psychotropic agents, patients in this study were on numerous types of drugs. Some of them were on combination antipsychotic agents and neuroleptics or sedatives. Eight subjects were on diazepam, 3 subjects were on carbamazepine, 5 were on fluoxetine, 2 were on promethazine, and 2 were using topiramate. In addition to that, extensive use of trihexphenidyle, an anticholinergic, was observed in this study. It was used in 42 out of 51(82.3%) patients regardless of dosage form or generation (2nd or 1st) of antipsychotic drug they were on. We studied in details the combination therapy patients were on; data are lengthy and not shown here.
However, a Kruskal–Wallis Test was used to determine if there was a significant difference between patients who were on different number of drugs and CGI-S levels. There was a significant difference between CGI-S normal group and CGI-S severe symptoms group, p=0.02, due to number of drugs used, as shown in Table 3 below.
| CGI-S levels | X2 | Sig | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal | Border- line | Mildly | Moder- ately | Markedly | Severally | |||
| 2.4 ÃÂ?± 1.27b | 0 ÃÂ?± 0 ab | 4.20ÃÂ?± 1.03ab |
4.33 ÃÂ?± 1.14ab | 3.40 ÃÂ?± 1.14ab | 4.32 ÃÂ?± 0.95ac | 11.687 | 0.02 | |
| Note: Different letters within row indicate a significant difference at the level 5% (0.02 p = 0.05), the value represent means ÃÂ?± SD |
||||||||
Table 3: Comparison of number of all drugs (psychotropic and non-psychotropic agents) and CGI score, using Kruskal Walls Test.
Moreover, we analyzed the use of trihexphenidyl among our patients as a function of number of psychotropic or non-psychotropic drugs used. Extensive use of which denotes either suffering of overwhelming side effects or deterioration of the patient condition. It might hinder quality of life or assessment of patient clinical situation, mask tardive dyskinesia and other long term side effects, and blurred line between clinical deterioration, progression of condition, drug-drug interactions, memory compromising and perception impairment.
Mann-Whitney test was used where we found a significant difference due to number of all drugs used and the use of trihexphenidyl, p=0.001. Also there was a significant difference between number of antipsychotic drugs used and thrihexphenidyl use, p=0.001, as show in C below (Table 4).
| Trihexyphenidyl_ used | Trihexyphenidyl- used | Mann-Whitney U | significance | |
|---|---|---|---|---|
| No. of all drugs | 4.22 ÃÂ?±1.037 | 2.50ÃÂ?± 1.5 | 79.00 | 0.001 |
| No. of psychiatric drugs |
2.80ÃÂ?±0.749 | 1.8ÃÂ?±0.789 | 79.00 | 0.001 |
| Note: The value represents means ÃÂ?± SD | ||||
Table 4: Differences between the total number of drugs and number of psychotropic drugs in regard to trihexyphenidyl use
One of the main objectives of this study is to measure the effect of dosage form on drug use among subjects of this study. Kruskal–Wallis Test showed a significant difference between different dosage forms of antipsychotics and number of all drugs, p=0.007 (Table 5).
| Oral drugs | *Combination dos- age forms | IM | ?2 | Sig. |
|---|---|---|---|---|
| 3.5 ÃÂ?± 1.291 | 4.11 ÃÂ?± 1.125 | 1 ÃÂ?± 0.00 | 9.843 | .007 |
| Note: The values represent means ÃÂ?± SD. *Combination dosage forms: oral and IM: intramuscular | ||||
Table 5: The comparison between type of dosage form used and number of all drugs
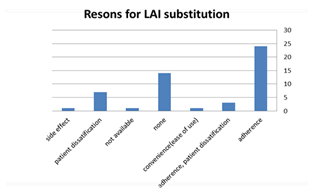
Patients were prescribed LAIs for the following reasons: non-adherence (47%), no reason at all (27.4%), patient dissatisfaction (13.7%), adherence and patient dissatisfaction (5.8%), side effects, convenience (ease of use), and availability of drug, (1.9%), for each.
We also studied separately the main reasons patients were prescribed LAIs from prescriber’s point of view in the 2 locations we visited. We found that; LAIs was added to regimen or patients were switched to for these reason; adherence (24 patients), for no reason at all (14), patient dissatisfaction (7), adherence and patient dissatisfaction (3), side effects, convenience (ease of use), and availability of drug, (1 patient) for each, Figure 1.
Figure 1: Percentage of patients who were switched to LAIs per reason of change.
Discussion
Most schizophrenic patients re-admitted to the hospital have demonstrated a degree of non-compliance (non-adherence), and it is often unclear whether or not non-compliance precedes relapse. Using LAIs will allow physicians to confirm relapse due to non-compliance despite appropriate medications. This obviously has important implications for post-treatment planning regarding the potential need for dose change, medication, and psychosocial therapy.
When patients receive LAIs there is certainty about a critical component in disease management. If the patient misses the injection, there is an immediate awareness on the part of the clinical team of the need for intervention, yet there is also some time to act before the crisis is likely to occur.
This was not applied on the subjects we studied. There was no clear measures for close follow up. LAIs were given according to availability. Often time, erratic drug supply, shortage of these agents, patients` ability to come and collect their medication or taking the injection and family support, play major role in commitment for LAIs intake.
Misuse of materials strongly predicts no adherence to the drug [10,11]. In cases of drug abuse, knowledge that the antipsychotic medication has definitely been taken, in the form of LAI, is important in determining the cause of possible subsequent relapse.
Family relationships often suffer when the uncertainty and anxiety associated with the possibility of non-compliance and its consequences greatly influence interactions. Many relatives and caregivers are directly involved in taking oral medications. Since they often face the initial burden (and even the threat and physical danger) associated with psychotic relapse, they are particularly sensitive to the issue of compliance. Using LAI medications can provide tremendous relief from this anxiety and facilitate the normalization of family interactions.
In our study, it was obviously clear the extensive use of trihexphenidyle which implies the majority of patients were suffering of whole spectrum of EPS and other medication related side effects. In addition, 39% of patients were using other non-psychotropic agents either to deal with side effects or to control prognosis of the condition regardless of their use of trihexphenidyle, and most often in addition to it.
Many reports evaluated predictors and risk factors for non-compliance. Despite these efforts, clinicians in routine clinical practice often cannot predict patients in peril at risk or are ready to identify patients who have already failed to adhere to their treatment regimen. Studies showed that patients and clinicians alike overestimate the degree of commitment [12,13]. Therefore, we must ask ourselves, given the provision of strategies to create the potential benefits of continuing medications, so why don’t we take the foremost of ourselves and our patients from this potential advantage? In other words, what are the barriers to describing LAI?
Shortage of supply for LAIs, intermittent supply, irregular physician’s visits, relapse, absence of clear routine for follow up and financial constraints were major barriers for prescribing LAIs among our patients.
Because psychopathology and social functioning can be exacerbated by repeated psychotic attacks in schizophrenia patients [14,15], relapse prevention is critical. There is strong evidence for the effectiveness of antipsychotics to prevent relapse in patients with chronic and first episode [16,17], in that the risk of relapse is 2 to 6 times higher without medication [16-19].
However, since non-compliance rates of up to 50% can limit the effectiveness of drug therapy [20,21], the use of long-term injectable antipsychotics (LAIs) is an important option [22]. In practice, patients and clinicians are sometimes reluctant to use LAIs due to stigma, needle pain, time restrictions, and side-effect and cost concerns [23].
This comes along with our results were 24 patients (47%) were non-complaint to their psychotropic medication the reason why they were switched to LAIs.
Given these barriers to the use of LAIs, convincing data is needed to demonstrate LAIs superiority of oral antipsychotics (OAPs) to support the use of LAIs. A meta-analysis found that LAIs were associated with a significantly lower relapse rates from OAPs [24]. There is ample evidence that antipsychotic medications significantly reduce the risk of relapse [25-27], however, and prevent neurotoxicity, even partial of non-adherence to oral medications may erode this potential benefit [26,28].
We could not assess the importance and validity of LAIs in prevention of relapse because of the intermittent use of these drugs and the absence of certain protocol for their use. However, the only one patient who was consistently on LAIs was doing fine on all three scales we used.
Our study also showed a significant relationship between the number of drugs used in general and the severity of symptoms on CGI-S scale where patients who were on 2 drugs in average were doing almost normal comparing to those who were using 4 drugs or more (severely ill).
In order to investigate the issue of improvement and where it stemmed from, we studied the effect of the dosage form. This will shed the light on the role of LAIs whether or not they were main reason for improvement and stability of patients.
We did that in two stages; first we analyzed the overall status of patients using the three scales as shown in Table 2 where patients who were on LAIs alone or as part of combination therapy, did better on GAF score and DEIPSS scales. They didn`t do better on severity of illness CGI-S scale.
We can`t blame that for the use of LAIs of course, rather to the delay of their use till last moment where the patients’ situation is worsening. We could infer that from the large number of drugs patients in this category were on. Patients were on LAIs continuously along with other conventional therapy. However, the degree for their commitment and the availability of LAIs were not addressed neither confirmed in this study for many reasons. We didn`t follow up patients prospectively, rather we only assessed their situation at the point of care during this study. Also there was no record of adherence or follow up.
The second level of analysis focused on the difference between the three categories of patients and dosage form used. In fact there was a significant difference between patients on IM dosage form and other dosage forms in term of how many drugs they needed.
Relapse cases were seen in the tow centers but to lesser extent than one might expect from their severity of illness as shown on CGI-S scale. Twenty tow patients (2 on oral dosage form and 20 on combination therapy) scored 6 on CGI-S scale, yet they doing well on GAF functionality score as shown in Table 2. On GAF, 33 patients, either on combination therapy or on LAIs alone had highest score levels (71-100) regardless of severity of illness or side effects. This could be attributed to the use of LAIs among them. This also might prove valid the nature of improvement was psychosocial rather than clinical among these patients having in mind that most of subjects in our study were basically on same set of antipsychotic agents and that all antipsychotics(first or second generation, LAIs or Oral) are equally effective.
Despite the plethora of studies that focused on the superiority of LAI over oral dosage form, it wasn`t proven so [29-31]. However, many studies proved the role of LAIs in improving adherence (either after first episode use or for maintenance purposes). These studies proved also LAIs value in preventing morbidity, worsening of symptoms, relapse and hospitalization [32-43].
The impact of non-adherence on symptoms severity and on the increasing tendency for use of polypharmacy and multiple dosage forms of same class or another class of equally effective antipsychotic agents was clearly explored in this study.
Having this said, LAIs didn`t improve markedly the overall clinical picture of schizophrenic patients, rather, they improve their functionality and psychosocial performance.
This subtle improvement was obvious despite of severity of symptoms and side effects among subjects in this study. We need more studies to address psychosocial aspects of improvement due to LAIs in randomized control trials.
Limitation of Study
Small sample size due to interruption of the project during the era of COVID-19 pandemic didn`t allow for more subjects to be investigated and limited our ability to generalize results stemmed from this study.
Low awareness of patients and sometimes their families of the importance of taking medication on time was major barrier of adherence and main cause of relapse. Dealing of the medical system with LAIs as an emergency for calming down aggressive and violent patients eroded any chance for long term assessment of the impact of LAOs on QoL.
Inability to assess adherence or relapse due to interrupted patient’s visits to the 2 centers either due to arresting them by police, being in seclusion, or isolated and detained by family. Other reasons such as; unavailability of transportation( these people are living at the margin of the community), financial issues, no close follow up from medical team or family, neither systematic profiling or contact information were also major barrier in continuity of care.
Conclusion
Adherence to psychotropic drugs is the mile stone in improving quality of life of schizophrenic patients. It stands behind stability of the condition, preventing relapse, improving functionality and minimizing hospitalization. LAI is a key dosage form in improving adherence and maintaining functionality. There were a high percentage of non-adherences among subjects of this study. Shortage of drug supply during COVID-19 pandemic exposed patients and their families. The imminently volatile political situation and subtle health system in our country erode all aspects of improvement for these vulnerable patients.
Ethical Consideration
A permission to run this study was granted first by the graduate office at the University, and then permission from the minister of health, Dr. Mia Kaileh was granted to get access to patients’ profiles at the ministry hospitals and mental health care centers and clinics.
Conflicts of Interest
We declare no conflict of interest for this research.
Author Contribution
Muamar shaheen: proposal of research, field organization and supervision, logistics and coordination, writing the manuscript, and data sorting and analysis.
Sara, Ibrahim, Hamza, and Basema: literature review, data collection and filed work.
Manar Al Junaidi: SPSS analysis.
Funding
We didn`t receive any fund for this study from anyone.
Acknowledgement
We would like to thank the staff at the mental health clinics and hospitals we visited for being so cooperative and enthusiastic. Special thanks for the attending physicians at both sites and for the ministry of health for making this project possible.
References
- J. M. Kane, E. Aguglia, A. C. Altamura, G. Ayuso, J. L. Brunello, et al. Guidelines for depot antipsychotic treatment in schizophrenia, Eur Neuropsychopharma- col, 8 (1998),55–66.
- J. M. Kane, F. Quitkin, A. Rifkin, J. R. Ramos-Loren- zi, D. V. Nayak. Fluphenazine versus placebo in pa- tients with remitted acute first episode schizophrenia, Arch Gen Psychiatry,39(1982):70–3
- T. J. Crow, J. F. MacMillan, A. L . Johnson, E. C. John- stone, A randomised controlled trial of prophylactic neuroleptic treatment, Br J Psychiatry, 148(1986),120– 7.
- D. G. Robinson, M. G. Woerner, H. M. Delman, J. M. Kane, Pharmacological treatments for first-episode schizophrenia, Schizophr Bull, 31(2005),705–22.
- D. G. Robinson, M. G. Woerner, J. M. J. Alvir, S. Geis- ler, A. Koreen, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder, Am J Psychiatry 156 (1999),544–9.
- C. M. Harding, J. Zubin, J. S. Strauss, Chronic- ity in schizophrenia: Revisited. Br J Psychiatry 161(1992),(suppl 18),27–37.
- M. Harrow, T. H. Jobe, Factors involved in outcome and recovery in schizophrenia patients not on antipsy- chotic medications: A 15 year multifollow-up study, J Nerv Ment Dis 195 (2007),406–14.
- J. M. Kane, M. Woerner, P. Weinhold, J. Wegner, B. Kinon. A prospective study of tardive dyskinesia devel- opment: Preliminary results, J Clin Psychopharmacol 2 (1982),345–9.
- American diabetes association, american psychiatric association, american association of clinical endocri- nologists, north american association for the study of obesity. Consensus development conference on anti- psychotic drugs and obesity and diabetes, J Clin Psy- chiatry 65 (2004), 267–73.
- W. S. Fenton, C. R. Blyler, R. K. Heinssen. Deter- minants of medication compliance in schizophrenia: Empirical and clinical findings, Schizophr Bull 23 (1997),637–51.
- J. P. Lacro, L. B. Dunn, C. R. Dolder, S. G. Leckband, D. V. Jeste. Prevalence of and risk factors for medica- tion nonadherence in patients with schizophrenia: A comprehensive review of recent literature, J Clin Psy- chiatry 63(2002),892–907.
- D. I . Velligan, F. Lam, L. Ereshefsky, A. L. Miller. Psychopharmacology: perspectives on medication ad- herence and atypical antipsychotic medications, Psy- chiatr Serv 54(2003),665–7.
- DI. Velligan, M. Wang, P. Diamond, DC. Glahn, D. Castillo, Bendle S, et al. Relationships among subjec- tive and objective measures of adherence to oral anti- psychotic medications,Psychiatr Serv,58(2007),1187– 92.
- J. A. Lieberman, D. Perkins, A. Belger. The early stag- es of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry, 50,(2001),884–897.
- N. E. van Haren, H. E. Hulshoff Pol, H. G. Schnack. Focal gray matter changes in schizophrenia across the course of the illness: A 5-year follow-up study. Neuro- psychopharmacology. 32(2007),2057–2066.
- D. Robinson, M. G . Woerner, J. M. Alvir. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry,56(1999),241–247.
- S. Leucht, TR. Barnes, W. Kissling, RR. Engel, C. Cor- rell, JM. Kane. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic re- view and exploratory metaanalysis of randomized, con- trolled trials. Am J Psychiatry,160(2003),1209–1222.
- S. Leucht, D. Arbter, R. R. Engel, W. Kissling, J. M. Davis. How effective are second-generation antipsy- chotic drugs? A meta-analysis of placebo-controlled trials,Mol Psychiatry,14(2009),429–447.
- S. Leucht, M. Tardy, K. Komossa. Antipsychotic drugs versus placebo for relapse prevention in schizophre- nia: A systematic review and meta-analysis. Lancet. 379(2012),2063–2071.
- C. R. Dolder, J. P. Lacro, L. B. Dunn, D. V. Jeste. An- tipsychotic medication adherence: is there a difference between typical and atypical agents?,Am J Psychia-try,159 (2002),103–108.
- D. I. Velligan, M. Wang, P. Diamond. Relationships among subjective and objective measures of adherence to oral antipsychotic medications, Psychiatr Serv. 58 (2007),1187–1192.
- J. M. Kane, C. Garcia-Ribera. Clinical guideline rec- ommendations for antipsychotic long-acting injec- tions, Br J Psychiatry Suppl. 52(2009),S63–S67.
- L. Waddell, M Taylor. Attitudes of patients and mental health staff to antipsychotic long-acting in- jections: Systematic review,Br J Psychiatry Suppl. 52(2009),S43–S50.
- C. Leucht, S. Heres, J. M. Kane, W. Kissling, J. M. Davis, et al. Oral versus depot antipsychotic drugs for schizophrenia: A critical systematic review and me- ta-analysis of randomised long-term trials,Schizophr Res. 127(2011),83–92
- R. Tandon, H. A. Nasrallah, M. S Keshavan. Schizo- phrenia, “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res. 122(2010),1– 23.
- N. R. Schooler. Relapse prevention and recovery in the treatment of schizophrenia. J Clin Psychiatry. 67(2006),19–23.
- J. M. Kane, C. U. Correll. Past and present progress in the pharmacologic treatment of schizophrenia,J Clin Psychiatry. 71(2010),1115–1124.
- J. M. Kane, T. Kishimoto, Correll C. U. Non-adher- ence to medication in patients with psychotic disor- ders: epidemiology, contributing factors and manage- ment strategies, World Psychiatry. 12(2013),216–226.
- C. Leucht, S. Heres, J. M. Kane, W. Kissling, JM. Da- vis, et al. Oral versus depot antipsychotic drugs for schizophrenia: A critical systematic review and me- ta-analysis of randomised long-term trials,Schizophr Res. 127,(2011)83–92.
- T. Kishimoto, A. Robenzadeh, Leucht C. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: A meta-analysis of randomized tri- als, Schizophr Bull. 40(2014),192–213.
- N. Y. Kirson, P. J. Weiden, S. Yermakov. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: Synthesizing results across different re- search designs. J Clin Psychiatry. 74 (2013),568–575.
- Kishimoto T, Agarwal V, Leucht S, Kane JM, Correll CU. Relapse prevention in schizophrenia: A systematic review and meta-analysis of second-generation anti- psychotics versus first-generation antipsychotics. Mol Psychiatry, 143(2011)1–14.
- T. Kishimoto, M. Nitta, M. Borenstein, J. M. Kane, Correll C. U. Long-acting injectable versus oral anti- psychotics in schizophrenia: A systematic review and meta-analysis of mirrorimage studies. J Clin Psychia- try. 74(2013),957–965.
- W. S. Fenton, C. R. Blyler, R. K. Heinssen. Deter- minants of medication compliance in schizophre- nia: Empirical and clinical findings. Schizophr Bull. 23(1997),637–651
- J. C. West, J. E. Wilk, M. Olfson. Patterns and quality of treatment for patients with schizophrenia in routine psychiatric practice. Psychiatr Serv,(2005),283–291.
- D. Robinson, M. G. Woerner, J. M. Alvir. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 56(1999),241–247.
- B. L. Svarstad, T. I. Shireman, J. K. Sweeney. Using drug claims data to assess the relationship of medica- tion adherence with hospitalization and costs. Psychi- atr Serv. 52(2001),805–811.
- P. J. Weiden, C. Kozma, A. Grogg, J. Locklear. Partial compliance and risk or rehospitalization among Cali- fornia Medicaid patients with schizophrenia. Psychiatr Serv. 55(2004),886–891.
- A. F. Lehman, J. Kreyenbuhl, R. W. Buchanan. The schizophrenia patient outcomes research team (PORT): Update treatment recommendations 2003. Schizophr Bull. 30(2004),193–217.
- M. Valenstein, L. A. Copeland, R. Owne, F. C. Blow, S. Visnic. Adherence assessments and the use of depot antipsychotics in patients with schizophrenia, J Clin Psychiatry. 62(2001),545–551.
- P. J. Weiden, N. R. Schooler, J. C. Weedon, A. El- mouchtari, A. Sunakawa, et al. A randomized con- trolled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome, J Clin Psychiatry, 70(2009),1397–1406.
- P. J. Weiden, N. R. Schooler, J. C. Weedon, A. El- mouchtari, A. Sunakawa-McMillan. Maintenance treatment with longacting injectable risperidone in first-episode schizophrenia: A randomized effective- ness study. J Clin Psychiatry. 73(2012),1224–1233.
- J. Tiihonen, J. Haukka, M. Taylor, P. M. Haddad, M. X Patel, et al. A nationwide cohort study of oral and de- pot antipsychotics after first hospitalization for schizo- phrenia. Am J Psychiatry. 168(2011),603–609.