Research - Journal of Drug and Alcohol Research ( 2024) Volume 13, Issue 6
Andrographolide Phytosomal Formulation for Enhanced Cognitive Resilience in Alzheimerâs Disease Model
Kottapalli Vinay Kumar1* and Kavitha N. Chilaka22Department of Pharmacology, Sir C. R. Reddy College of Pharmaceutical Sciences, India
Kottapalli Vinay Kumar, Department of Pharmacology, GITAM School of Pharmacy, GITAM (Deemed to be University), India, Email: vkottapa@gitam.in
Received: 29-May-2024, Manuscript No. DAR-24-134219; Editor assigned: 31-May-2024, Pre QC No. JDAR-24-134219 (PQ); Reviewed: 14-Jun-2024, QC No. JDAR-24-134219; Revised: 19-Jun-2024, Manuscript No. JDAR-24-134219 (R); Published: 26-Jun-2024, DOI: 10.4303/JDAR/236305
Abstract
Alzheimer’s Disease (AD) is one of the common forms of dementia seen in elderly people. The present study was aimed at developing Andrographolide Phytosomes (AGP) and comparing it with the Andrographolide (AG) on scopolamine-induced Alzheimer’s disease by assessing cognitive function and biochemical markers. The Phytosomes confirmed characterization process FITR, DSC, Zeta potential, % Entrapment Efficiency and Scanning Electron Microscopy. AGP treatment significantly decreased the escape latency (p<0.01**) and improved conditioned avoidance response (p<0.001***) compared to AG. Pre-treatment with AGP significantly decreased acetylcholine esterase levels (p<0.01**) and increased hippocampal catalase (p<0.01**), superoxide dismutase (p<0.001***) and reduced glutathione levels (p<0.001***) which aligns with the results observed in the standard group when compared AG group indicating a preventive strategy against the progression of AD. This approach of AGP provides the potential therapeutic application in human neurodegenerative disease in future.
Keywords
Alzheimer’s Disease (AD); Andrographolide Phytosomes (AGP); Andrographolide (AG); Scopolamine; Antioxidant/oxidant system; Acetylcholine esterase
Introduction
Alzheimer’s Disease (AD) is a chronic neurological condition characterised by a gradual decrease in memory function [1]. It is characterised by the presence of cerebral oxidative stress, which is accompanied by the degeneration of cholinergic neurons in the basal forebrain and hippocampus [2,3]. The activation of cholinergic neurons in the central nervous system has a significant impact on the processes like learning and memory [4]. The process of memory formation involves the activity of several neurotransmitters and neural pathways [5]. The presence of cognitive impairments in AD is linked to functional deficiencies in the cholinergic system [6]. Scopolamine, a potent antagonist of muscarinic cholinergic receptors, induces amnesic effects in laboratory animals. The use of scopolamine-induced amnesia as an experimental animal model has been utilised extensively to evaluate drugs that are expected to have therapeutic benefits in treating dementia [7]. Scopolamine disrupts cholinergic transmission in the central nervous system, resulting in cholinergic dysfunction and memory deficits in rats [8].
Acetyl cholinesterase inhibitors, especially, rivastigmine, galantamine and donepezil, are the most potent and clinically approved pharmacotherapeutic treatments for cognitive impairment [9]. Donepezil is a potent inhibitor of acetylcholinesterase and butyrylcholinesterase [10]. Despite the advances in research, currently available drugs are not optimal for clinical use due to their undesirable side effects [11,12]. Therefore, it is necessary to explore alternative for amnesia treatment. Currently, medicinal plants have been used mostly for their efficacy in treating cognitive problems [13].
Andrographolide (AG), a diterpenoid found in the leaves of Andrographis paniculata, a member of the Acanthaceae family, has long been employed in traditional medicine to treat a variety of illnesses in humans. It exhibits several actions including antioxidant, anti-inflammatory, neuroprotective and anti-cancer characteristics. AG protects the brain from oxidative stress caused by nicotine and against brain ischemia induced neurotoxicity and inflammation related neurodegeneration [14,15]. The therapeutic applicability of AG is limited due to its instability and poor water solubility, resulting in low bioavailability.
Phytosomes are more advantageous for the high capacity of drug encapsulation, improved stability due to the formation of chemical interactions between the polar head of the amphiphile molecule and phytoconstituent and enhanced bioavailability [16,17]. A higher rate of absorption results in a reduced number of active components needed to produce a biological effect, including polar phytoconstituents. Therefore, andrographolide phytosomes were prepared and evaluated for their anti alzheimers activity on scopolamine induced dementia in mice.
Materials and Methods
Preparation of andrographolide phytosomes
The AGP are developed by the solvent evaporation process employing different AG: Soya Lecithin ratios of 1:0.5, 1:1, and 1:1.5. The specified amount of AG and SL is dissolved in a small volume of methanol in separate containers. The AG and SL solutions are combined in a round bottom flask and subjected to reflux for a duration of one hour at a temperature of 65°C, utilising an air reflux condenser. Once the reflux is finished, the solvent is extracted using a rotary evaporator. Characterization of phytosomes has been established by the use of analytical techniques.
Fourier transform infrared spectroscopy
The molecular interactions of AG, SL, and AGP were examined using an FTIR spectrophotometer (Alpha Bruker). The samples were subjected to a drying process at a temperature of 50°C in order to eliminate any potential impact of moisture. The samples were scanned throughout a range of wavelengths from 4000 cm-1 to 400 cm-1.
Differential scanning calorimetry
The thermograms of AG, SL, and AGP were obtained utilising a Differential Scanning Calorimeter (TA DSC Q20). The solid and semi-solid samples were examined by placing a 5 mg sample in aluminium pans and heating it from 30°C to 350°C at a heating rate of 10°C/Min under a nitrogen flow rate of 15 ml/min.
Drug content
The AG content of the produced phytosome formulation was assessed by diluting 100 mg of the sample in 10 ml of water, then subjecting it to centrifugation and further dilutions. The dilutions were examined using a UV-Visible spectrophotometer (PCI Analytics) at a wavelength of 236 nm.
Particle size and zeta potential
The AGP particle size was analysed using a laser particle size analyzer (Horiba SZ-100). Initially, a 10 mg AGP was evenly distributed in 10 ml of distilled water with the use ultrasonic waves for a duration of 1 minute. The samples were examined for particle size within a sensitivity range of 1 nm to 10 μm, and for charge within a range of -200 millivolts to +200 millivolts.
% Entrapment efficiency
The entrapment efficiency of the AGP was assessed by dispersing 10 mg of the AGP in 10 ml of distilled water, then subjecting it to brief sonication and centrifugation at 16,000 rpm for 30 minutes to separate the phytosomes from the un entrapped drug. Supernatant, was collected and adequately diluted for the purpose of analysing the concentration of the AG. This analysis was conducted using a UV spectrophotometer (PCI Analytics) set to a wavelength of 236 nm.
The % EE was calculated as follows

Scanning electron microscopy: Structural features of AGP analysed using a scanning electron microscope (JEOL JSM).
Experimental design for the estimation of anti-alzheimer activity of AGP on Scopolamine (SCO) induced AD
Animals and treatment: Mice weighing 20 g–23 g were purchased from vyas labs hyderabad. The mice were housed in individual cages under controlled temperature (20°C ± 2°C), 12-h light/dark cycle, and specific humidified conditions (50% ± 10%). All mice were provided ad libitum access to water and a standard diet. The Institutional Animal Ethical Committee (IAEC) of Sir C.R. Reddy College of Pharmaceutical Sciences, Eluru, India, with protocol number SCRRCOPS/IAEC/2023/1.11. approved the animal protocol used in the present study. After one week of acclimatization, the mice (n=40) were randomly divided into 5 groups (n=8): Negative control (Saline treatment group); Positive control: SCO (1 mg/kg. I.P for 14 days); treated group; Donepezil DNP (0.5 mg/ kg, p.o) +SCO, AG (50 mg/kg. I.P) +SCO; AGP (50 mg/ kg. I.P) +SCO group. SCO was dissolved in 0.9% sodium chloride (NaCl) and administered by I.P injection to mice. The behavioural experiments were conducted 30 min after SCO injection.
Morris Water Maze (MWM) test
The Morris Water Maze (MWM) test is frequently used to evaluate spatial learning and memory in animal models [18,19]. The water maze consisted of a circular pool measuring 1.0 m in diameter and 0.38 m in height. The collection was filled with black ink at a temperature of (23°C ± 2°C), and reached a depth of 25 cm. The platform stayed immobile during the whole training period. A harmless dye was added to the water to make the platform invisible, causing it to become opaque. The study involved conducting memory acquisition sessions, with each session lasting 120 seconds, twice a day for a continuous period of 4 days. Each trial had a minimum interval of 15 minutes. During each acquisition experiment, the mice were given complete freedom to move around in the water and actively search for the hidden platform. Upon correctly identifying the platform, it was permitted to stay on it for an additional 20 seconds for the purpose of resting. If a mice failed to reach the platform within 120 seconds, it was gently guided towards it and then kept on the platform for a duration of 20 seconds. The researchers calculated the average escape delay by monitoring the duration it took for each mouse to locate the hidden platform. This measure was employed to assess the mice acquisition or learning capabilities.
Cook’s Pole Climbing (CPC) test
The mice learning and memory were assessed by evaluating their conditioned avoidance response using the Cooks pole climbing device [20]. The ground component comprises grid rods that function as a trigger for shock. Initially, mice were individually taught and their performance was observed during the acquisition phase. The retention trial was conducted on the 0th, 7th, and 14th days and the results were recorded. The evaluation process took into account a cut-off period of 120 seconds [21]. Mice that had received training were administered saline, AG, AGP, or DNP, and their conditioned avoidance responses were evaluated.
Collection of brain hippocampus
At the completion of the behavioural test, the mice were euthanized by cervical decapitation while under light anaesthesia. Following decapitation, the brain was promptly extracted, weighed, and rinsed with isotonic saline solution. The hippocampus was carefully separated and homogenised in a 10% (w/v) concentration using a 0.1 M phosphate buffer at pH 7.4. The homogenates, with a concentration of 10% weight/volume, were centrifugation at 10,000 g for 15 minutes to separate the supernatant. The resulting cloudy supernatant liquid was utilised to assess the biochemical parameters of the hippocampus [22].
Brain hippocampus chemical parameters
AChE activity (expressed as nmol/min/mg protein) was tested according to the method adopted by Ellman, et al. (1961), while Catalase (CAT) (expressed as n moles of H2O2 consumed/minute/mg protein) by Aebi method, Superoxide Dismutase (SOD) (expressed as units per mg of protein) by Nishikimi method, reduced Glutathione (GSH) (expressed nmol/mg/Protein) by Beulter method [23-26].
Statistical analysis
Data were expressed as means ± S.E.M., and comparisons between means were carried out using one way Analysis of Variance (ANOVA) followed by Dunnets test. A probability level of less than 0.05 was accepted as being significant in all types of statistical tests. Scopolamine-induced dementia significantly reduced the condition.
Results
Preparation of andrographolide phytosomes
The AG phytosomes were successfully prepared by the solvent evaporation technique. The thin film of phytosomes were collected from the round bottomed flask and are stored in a dessicator until further use. The yield of the phytsomes was found to be 93.5%, 95% and 96% for 1:0, 1:1 and 1:1.5 ratios respectively.
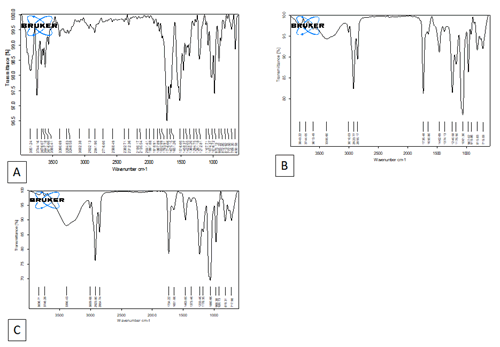
FT IR analysis: The FTIR spectrum shows the following characteristics for Andrographolide a O-H stretching vibration at 3390.69 cm-1, C-H stretching vibration from 2841.90-2932.13 cm-1, C=O stretching at 1716.66 cm-1, and C-O-C group absorption at 1212.81 cm-1 [27]. The SL has exhibited absorption at following wave numbers C–H stretching band vibration at 2923.77 cm-1 and 2855.17 cm-1, stretching band of ester carbonyl group at 1735.48 cm-1, and ester C–O stretching band at 1240.58 cm-1 [28]. The prepared AG Phytosomes exhibited the characteristic peaks O-H stretching vibration at 3390.43 cm-1, C-H stretching vibration at 2923.8 and 2854.74 cm-1, C=O stretching vibration at 1734.22 cm-1, and ester C–O stretching band at 1233.46 cm-1. The Phytosomes exhibited a shift of C-H stretching and C=O stretching. This might be taken as an evidence of intermolecular hydrogen bonding between andrographolide and soya lecithin components. These interactions are essential for AG loading and controlling its release (Figure 1).
Figure 1: FTIR Analysis, A) FTIR Spectrum of AG, B) FTIR Spectrum of Soya Lecithin, C) FTIR Spectrum of AGP
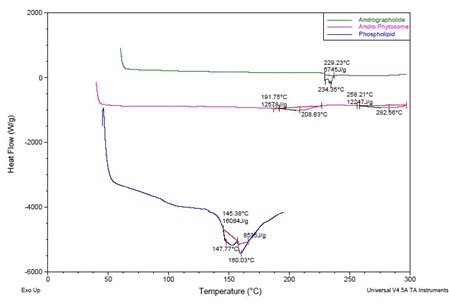
Differential scanning calorimetry: To further confirm the interaction between AG and SL, DSC thermograms were recorded for AG, SL and AGP. The AG thermogram displayed a melting point at 234.35°C, The SL exhibited slightly broad endothermic curves at 147.74 and 160.39°C which can be attributed to the amorphous nature of the substance [29,30]. The prepared AGP exhibited slightly broad endothermic curves at 208.83°C and 282.56°C the shift in the peak position might be taken as an evidence of hydrogen bonding between the drug and SL components, which correlates the results obtained from FTIR studies (Figure 2). These interactions are essential to facilitate drug loading in phytosome and release once it is administered in the body.
Figure 2: DSC, a) AD (Green colour), b) AGP (Pink colour), c) Phospholipid (Blue colour)
Drug content: The drug content of the prepared various ratios of Phytosomes 1:0.5, 1:1, 1:1.5 were observed % drug content 97.6 ± 0.53, 98.6 ± 0.35 and 97.2 ± 0.51 respectively.
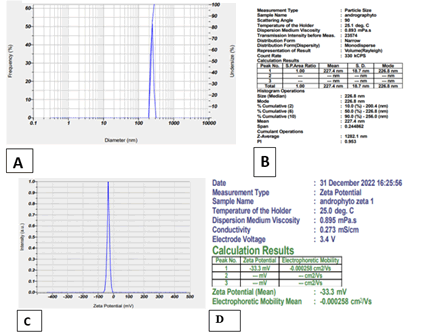
Particle size and zeta potential: The mean particle size of the optimal formulation 1:1 is found to be 227.4 nm. The zeta potential of the optimal formulation 1:1 was found to be -33.3 Mv (Figure 3).
Figure 3: A) Particle Size distribution of optimized formulations, B) Particle size of the optimized formulations, C) Zeta potential graph of the optimized formulation, D) Zeta potential value of optimized formulation
% Entrapment efficiency: The entrapment efficiency of the various formulation ratio 1:0.5, 1:1 and 1:1.5 was found to be as 65% ± 0.55%, 76% ± 0.45% and 71% ± 0.65% respectively.
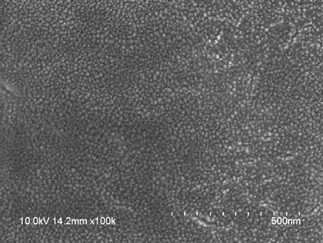
Scanning electron microscopy: The SEM reveals vesicular systems in a circular structure indicating the formation of Phytosomes (Figure 4).
Figure 4: SEM of AGP
Behavioural parameters
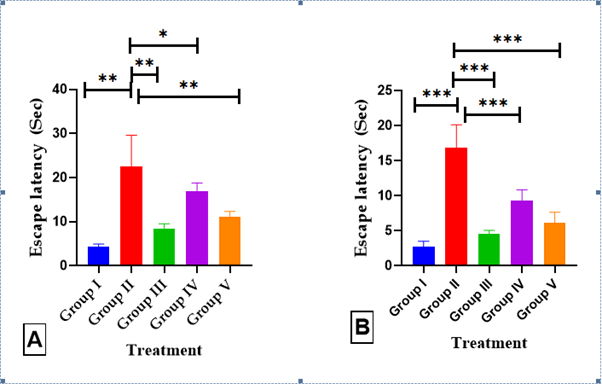
MWM test: The effects of AGP on long-term and spatial memory were measured using the MWM test (F (4,25=26), P<0.0001). The swimming time for mice to find the escape platform decreased in all groups except the scopolamineinduced mice during the experimental period. SCO-treated mice exhibited a significantly longer escape latency time of 22.50 ± 2.9 (p<0.001***) than the normal mice. These results demonstrated that scopolamine triggered the impairment of long-term and spatial memory. In comparison, the administration of AGP (50 mg/kg) showed a shorter 11.17 ± 0.48 (p<0.01**) escape latency time compared with that of the SCO treated mice. In particular AGP induced similar ELT to those of the everyday and DON 8.3 ± 0.49 (p<0.01***) groups (Table 1) (Figure 5).
Figure 5: Effects of AGP on the water escape time of mice with memory impairment induced by SCO, (A) MWM Escape latency test, (B) CPC Escape latency from shock. Data are expressed as means ± SEM, n=8 in each group. Statistically significance p<0.05*, p<0.01**, p<0.001***. Comparison with positive (SCO) control mice
Table 1: Effects of AGP on the water escape time of mice with memory impairment induced by SCO using MWM escape latency test
| Mice | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| M1 | 4.5 | 22 | 9 | 17 | 12 |
| M2 | 5 | 29 | 8 | 18 | 11 |
| M3 | 4 | 31 | 7 | 14 | 11 |
| M4 | 4 | 11 | 7 | 15 | 12 |
| M5 | 4 | 21 | 9 | 18 | 9 |
| M6 | 5 | 21 | 10 | 19 | 12 |
| Mean | 4.42 | 22.5 | 8.33 | 16.83 | 11.17 |
| SD | 0.49 | 7.09 | 1.21 | 1.94 | 1.17 |
| SEM | 0.2 | 2.9 | 0.49 | 0.79 | 0.48 |
CPC test: The escape latency time/conditional avoidance of each mouse during learning and its retention was screened by the CPC test (F (4.35=56.74), P<0.0001). During acquisition and 0th day, no significant difference in Escape latency time (ELT) was observed between Positive control mice and DON treatment groups. However, on 14th day considerable increase in the ELT was seen in the SCO mice 16.83 ± 1.35 (p<0.001***) as compared to the normal mice 2.67 ± 0.33 (p<0.001***), while treatment with DON, AGP (50 mg/kg) decreased the ELT 4.50 ± 0.22 (p<0.001***), and 6.17 ± 0.6 (p<0.001***), respectively, when compared to the SCO treated mice (Table 2) (Figure 5).
Table 2: Effects of AGP on the water escape time of mice with memory impairment induced by SCO using CPC test
| Mice | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| M1 | 2 | 23 | 4 | 8 | 8 |
| M2 | 3 | 17 | 5 | 9 | 4 |
| M3 | 2 | 16 | 5 | 10 | 6 |
| M4 | 4 | 14 | 4 | 8 | 7 |
| M5 | 3 | 17 | 5 | 9 | 5 |
| M6 | 2 | 14 | 4 | 12 | 7 |
| Mean | 2.67 | 16.83 | 4.5 | 9.33 | 6.17 |
| SD | 0.82 | 3.31 | 0.55 | 1.51 | 1.47 |
| SEM | 0.33 | 1.35 | 0.22 | 0.61 | 0.6 |
Brain hippocampus chemical parameters
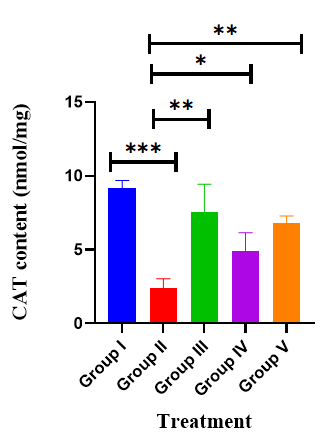
Effects of AGP on hippocampus CAT: Effect of AGP on brain hippocampus Catalase revealed by administration of SCO exhibited a significantly decreased catalase activity (2.37 ± 0.27, p<0.001***) when compared with the normal control mice (9.17 ± 0.22). Nevertheless, the administration of AGP 50 mg/kg, and DON significantly increased the catalase activity to 6.83 ± 0.19 (p<0.01**), 7.53 ± 0.78 (p<0.01**), 6.29 ± 0.37 (p<0.001***) respectively compared with the positive control mice (F (4,30)=41.4, p<0.001) (Table 3) (Figure 6).
Table 3: Effect of AGP on CAT activity
| Mice | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| M1 | 8.6 | 2.96 | 9.4 | 5.7 | 6.9 |
| M2 | 9.8 | 1.2 | 5.79 | 5.8 | 7.5 |
| M3 | 9.8 | 2.96 | 9.5 | 6.5 | 6.6 |
| M4 | 8.9 | 2.4 | 5.8 | 3.5 | 6.5 |
| M5 | 9.2 | 2.1 | 8.9 | 3.9 | 7.2 |
| M6 | 8.7 | 2.6 | 5.8 | 4.2 | 6.3 |
| Mean | 9.17 | 2.37 | 7.53 | 4.93 | 6.83 |
| SD | 0.53 | 0.66 | 1.91 | 1.22 | 0.45 |
| SEM | 0.22 | 0.27 | 0.78 | 0.5 | 0.19 |
Figure 6: Effects of AGP on hippocampus CAT. Data are expressed as means ± SEM, n=8 in each group. Statistically significance p<0.05*, p<0.01**, p<0.001***. Comparison with positive (SCO) control mice
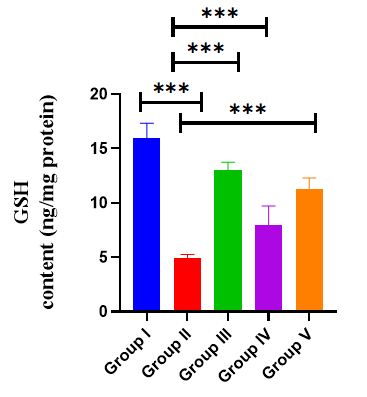
Effects of AGP on hippocampus GSH: Effect of AGP on brain hippocampus GSH revealed by administration of SCO exhibited a significantly (p<0.001***) decreased brain GSH activity to 0.493 ± 0.01, compared with the normal control mice (15.98 ± 0.54). Nevertheless, the administration of AGP 50 mg/kg, and DON significantly increased the GSH levels to 11.3 ± 0.40 (p<0.001***), 2.02 ± 0.29 (p<0.001***), 2.36 ± 0.30 (p<0.001***), respectively compared with the positive control mice (F (4,30)=86.6, p<0.0001) (Table 4) (Figure 7).
Table 4: Effect of AGP on GSH activity
| Mice | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| M1 | 17.5 | 4.6 | 14.3 | 8.9 | 10.4 |
| M2 | 15.4 | 5.4 | 12.6 | 9.1 | 11.2 |
| M3 | 13.8 | 4.6 | 12.4 | 9.1 | 10.4 |
| M4 | 16.1 | 5.1 | 13.2 | 5.2 | 13.1 |
| M5 | 15.9 | 4.8 | 12.6 | 6.3 | 11.5 |
| M6 | 17.2 | 5.1 | 13.2 | 9.2 | 11.2 |
| Mean | 15.98 | 4.93 | 13.05 | 7.97 | 11.3 |
| SD | 1.33 | 0.32 | 0.7 | 1.75 | 0.99 |
| SEM | 0.54 | 0.13 | 0.28 | 0.72 | 0.4 |
Figure 7: Effects of AGP on hippocampus GSH. Data are expressed as means ± SEM, n=8 in each group. Statistically significance p<0.05*, p<0.01**, p<0.001***. Comparison with positive (SCO) control mice
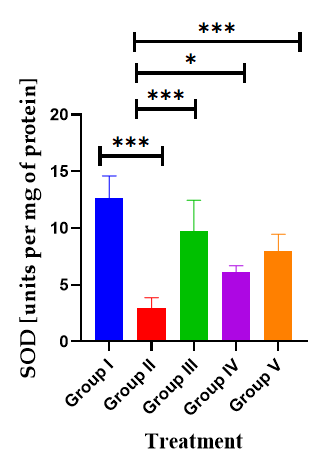
Effect of AGP on SOD activity: Effect of AGP on brain hippocampus SOD revealed by administration of SCO exhibited a significantly decreased brain SOD activity (2.90 ± 0.4, p<0.001***) compared with the normal control mice (12.62 ± 0.81). Nevertheless, the administration of AGP 50 mg/kg, and DON significantly increased the SOD activity to 7.97 ± 0.61 (p<0.001***), 9.71 ± 1.12 (p<0.001***), 6.53 ± 0.14 (p<0.001***) respectively compared with the positive control mice (F (4, 30)=26.9, p<0.0001) (Table 5) (Figure 8).
Figure 8: Effects of AGP on hippocampus GSH. Data are expressed as means ± SEM, n=8 in each group. Statistically significance p<0.05*, p<0.01**, p<0.001***. Comparison with positive (SCO) control mice
Table 5: Effect of AGP on SOD activity
| Mice | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| M1 | 13.5 | 1.57 | 14.66 | 6.5 | 7.2 |
| M2 | 12.8 | 3.2 | 6.4 | 5.14 | 6.33 |
| M3 | 14.7 | 2.54 | 10.2 | 6.3 | 6.5 |
| M4 | 10.2 | 4.2 | 9.2 | 6.5 | 9.8 |
| M5 | 14.3 | 3.7 | 8.5 | 5.9 | 8.7 |
| M6 | 10.2 | 2.2 | 9.3 | 6.5 | 9.3 |
| Mean | 12.62 | 2.9 | 9.71 | 6.14 | 7.97 |
| SD | 1.98 | 0.98 | 2.74 | 0.54 | 1.49 |
| SEM | 0.81 | 0.4 | 1.12 | 0.22 | 0.61 |
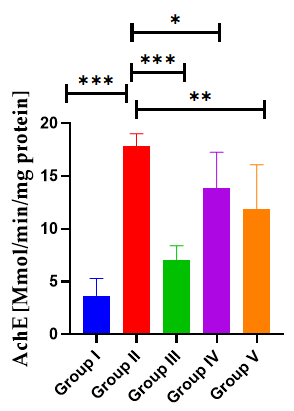
Effect of AGP on AchE activity: Effect of AGP on brain hippocampus AchE revealed by administration of SCO exhibited a significant rise in brain AChE activity (17.83 ± 0.67, p<0.001***) when compared with the normal control mice (0.051 ± 0.0098). Nevertheless, the administration of AGP 50 mg/kg, and DON significantly suppressed the AChE activity to 11.83 ± 1.74 (p<0.01**) and 7.00 ± 0.58 (p<0.01**), respectively compared with the positive control mice (F (5,30)=26.8, p<0.0001) (Table 6) (Figure 9).
Table 6: Effect of AGP on AchE activity
| Mice | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| M1 | 2 | 19 | 9 | 13 | 14 |
| M2 | 5 | 16 | 7 | 14 | 19 |
| M3 | 4 | 17 | 8 | 16 | 7 |
| M4 | 6 | 18 | 5 | 9 | 12 |
| M5 | 2 | 18 | 7 | 19 | 10 |
| M6 | 3 | 19 | 6 | 12 | 9 |
| Mean | 3.67 | 17.83 | 7 | 13.83 | 11.83 |
| SD | 1.63 | 1.17 | 1.41 | 3.43 | 4.26 |
| SEM | 0.67 | 0.48 | 0.58 | 1.4 | 1.74 |
Figure 9: Effects of AGP on hippocampus SOD. Data are expressed as means ± SEM, n=8 in each group. Statistically significance p<0.05*, p<0.01**, p<0.001***. Comparison with positive (SCO) control mice
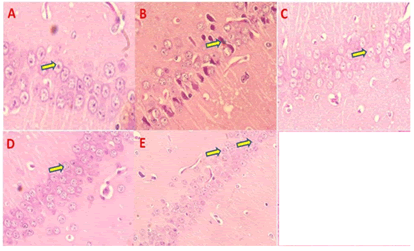
Histopathology studies: The control mice revealed no histopathological alterations, with typical histological features of the hippocampus CA1 regions showing intact pyramidal neurons in the brain hippocampus region. SCO treated mice group CA1 region contained dead cells confirmed by the appearance of dark, skinny, and pyknotic nuclei. However, AGP 50 mg/kg and standard DONtreated mice hippocampus CA1 were significantly reduced dead cells compared to treated mice on SCO treated mice (Figure 10).
Figure 10: Effect of AGP on CA 1 region, A) Normal control mice showed pyramidal cell with prominent nucleus and dense arrangement (Yellow Arrow), B) SCO treated mice hippocampus region showed loose arrangement with pyramidal cell with chromatid condensation, Apoptotic cells (Yellow Arrow), C) DON treatment normal pyramidal cell with prominent nucleus and dense arrangement (Yellow Arrow), D) AG treated mice hippocampus region showed pyramidal cell with chromatid condensation (Yellow Arrow), E) AGP treated mice hippocampus region-normal pyramidal cell with very less chromatid condensation, dense arrangement (Yellow Arrow)
Discussion
Neurodegenerative disorders, such Alzheimer’s Disease (AD), lead to impaired behaviour and cognitive function. These diseases pose significant health challenges and contribute to increased financial burden in nations with ageing populations [31]. Polyphenols, which are natural medicinal substances, have gained recent recognition as a potential alternative treatment for neurodegenerative illnesses [32]. The issue of bioavailability is a significant hurdle, often limiting the conversion of promising herbs into clinically effective medications. Phytosomes are an effective approach to address this limitation. Several studies have documented the enhanced therapeutic effectiveness resulting from the encapsulation of herbal medications with phytosomes [33-35]. Soya Lecithin is a constituent of the phytosome that has the potential to improve the stability and chemical connection between AG and phytosome. Multiple studies have documented that the utilisation of a phytochemical drug delivery method, such as phytosome, yields a favourable impact on the bioavailability of drugs, resulting in enhanced pharmacological activity for illnesses affecting the pulmonary and central nervous systems [36- 39]. The current work involved the development of AGP and the evaluation of its protective impact in AD rats, administered at a dose of 50 mg/kg body weight.
Scopolamine, a widely renowned anticholinergic medication, is frequently employed as a standard experimental treatment to deliberately produce cognitive impairment in live animal models [40,41]. Previous research has indicated that the administration of scopolamine led to a decline in cognitive function in the radial maze and hindered the acquisition process in the Morris water maze test [42]. This study aimed to validate the attenuating effects of AGP on scopolamine-induced learning and memory deficits in mice. To do this, the passive avoidance, and Morris water maze tests were conducted. Passive avoidance tests are based on the conflict between fear and preference for darkness and are performed to evaluate short-term learning and memory abilities in rats that are exposed to an unavoidable electric shock when entering a dark compartment [43].
In this study, the SCO group did not exhibit significant memory of the electrical shock they had experienced the previous day, and they were able to climb the pole practically quickly. Nevertheless, the administration of AGP significantly delayed the step-through latency. The administration of AGP successfully mitigated the decrease in step-through latency caused by scopolamine. The aforementioned results of the passive avoidance test demonstrate that AGP effectively prevents long-term impairments in avoidance memory in mice, in comparison to AG therapy.
The Morris water maze test is a commonly used technique for assessing the enduring spatial learning and memory abilities of animals in a controlled laboratory setting [36]. During the training trials, the time it takes to find the platform is an indicator of their ability to learn and remember the location of the hidden platform, which assesses their longterm spatial learning capacity. Throughout the course of the study, the time it took for all groups to reach the platform reduced steadily over a period of 5 days. However, the SCOP group exhibited a slower rate of learning compared to the other groups. Following the training session, the swimming duration inside the designated area where the platform was situated was recorded in order to assess the spatial memory capacity of the rats. The SCO group exhibited irregular and random swimming patterns throughout the Maze resulting in a significant (p<0.001) reduction in the time it took for the rats to cross the target zone, as compared to the control group. The decrease in performance of the SCO group indicated significant longterm cognitive damage in mice caused by SCO. The mice treated with AGP exhibited precise navigation to the target quadrant and demonstrated comparable swimming time in the target zone when compared to the mice treated with SCO and AG. The findings from the Morris water maze test indicate that AGP successfully reduces long-term impairments in spatial learning and memory.
Overall, the treatment of AGP successfully alleviated learning and memory impairment in mice induced by SCO. The findings from behavioural assessments, such as passive avoidance and Morris water maze tests, exhibited a similarity to previous investigations that have documented the amelioration of learning and memory deficits induced by SCO [40,41].
The cholinergic deficit is a major mechanism of Alzheimer’s disease that is linked to the decline of cognitive skills, such as memory loss [39]. Cholinergic transmission is mostly ended by acetylcholinesterase (AChE), an enzyme responsible for the breakdown of acetylcholine (ACh), a crucial neurotransmitter, in the cholinergic neurons [44]. Scopolamine has been found to stimulate an elevation in acetylcholinesterase (AChE) activity [32]. Studies by Kaur, et al. (2015) and Sharma, et al. (2019) have shown that AChE inhibitors enhance cognitive function in rats treated with SCO. The administration of AGP suppressed the elevation of AChE activity induced by SCO in the hippocampus collected following the behavioural tests in this investigation. According to Heo HJ in 2004, there is a correlation between reduced AChE activity in the brain and enhanced cognitive function. Therefore, the findings of this study indicate that AGP has a beneficial impact on learning and memory function, mostly by suppressing the activity of AChE in the hippocampus.
Oxidative stress induces injury to brain tissue, hence exacerbating impairments in learning and memory [38]. Aerobic organisms include a diverse array of internal antioxidant defence mechanisms, which include both enzymatic and non-enzymatic antioxidants. These defence systems serve to safeguard tissues from oxidative harm [40]. SOD functions as an antioxidant enzyme that controls oxidative stress by eliminating superoxide radical anions. The study found that the activity of the antioxidant enzyme SOD was considerably decreased in the SCO group compared to the control group that did not receive AGP or scopolamine treatment.
Nevertheless, the administration of AGP before to the experiment prevented a reduction in SOD, CAT and GSH activity in the hippocampus of mice produced by SCO. Consistent with the findings of this study, earlier research has shown that cognitive performance can be enhanced by the neuroprotective effects resulting from the decrease in oxidative stress through increased SOD, CAT and GSH activity activity [43,44]. The study indicated that AGP had anti-amnesic effects in in vivo behavioural testing. AGP acts as an exogenous antioxidant, supporting the antioxidant defence system and preventing oxidative damage in neurons.
Conclusion
This study aims to improve the antiamnesic efficacy of Andrographolides by developing a new phytoformulation. The results demonstrated the greater effectiveness of this formulation in comparison to AG by lowering both the oxidative burden and the inhibition of AchE. The precise mechanism underlying the higher therapeutic effectiveness of the formulation necessitates an examination of pharmacokinetic parameters to validate its heightened absorption and improved bioavailability.
Acknowledgement
Authors would like to acknowledge the support of our respective institutions, the GITAM Institute of Pharmacy, GITAM Deemed to Be University, Rushikonda, Visakhapatnam, Andhra Pradesh, India, and the Sir C.R. Reddy College of Pharmaceutical Sciences, Eluru, India.
Funding
This study was self-funded by the authors.
Ethics Approval
This study was approved by the Institutional Animal ethical Committee (IAEC) of Sir C.R. Reddy College of Pharmaceutical Sciences, Eluru, India, with protocol number SCRRCOPS/IAEC/2023/1.11.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Informed Consent
Not applicable. No human subjects were involved in this study.
Author Contributions
[Author 1 Kottapalli Vinay Kumar]: All aspects of the research, including conceptualization, methodology, investigation, data analysis, writing, and reviewing.
[Author 2 Kavitha N. Chilaka]: Research supervision and guidance.
Competing Interests
The authors declare that they have no competing interests.
References
- H.E. Abo Mansour, A.I. Elberri, M.E.S. Ghoneim, W.A. Samman, A.A. Alhaddad, et al. The potential neuroprotective effect of thymoquinone on scopolamine-induced in vivo Alzheimer's disease-like condition: Mechanistic insights, Molecules, 28(2023):6566.
- H. Aebi, Catalase in vitro, Methods Enzymol, 105(1984):121-126.
- J.A. Woolliams, G. Wiener, P.H. Anderson, C.H. McMurray, Improved method for the determination of blood glutathione, J Lab Clin Med, 61(1963):882.
- K. Blennow, M.J. de Leon, H. Zetterberg, Alzheimer's disease, Lancet, 368(2006):387.
- A Bloklan, Acetylcholine: A neurotransmitter for learning and memory? Brain Res Rev, 21(1996):285.
- S.J. Chan, W.S.F. Wong, P.T.H. Wong, Neuroprotective effects of andrographolide in a rat model of permanent cerebral ischemia, Br J Pharmacol, 161(2010):668.
- L. Cook, E. Weidley, Behavioral effects of some psychopharmacological agents, Ann N Y Acad Sci, 66(1957):740.
- N. Dewan, D. Dasgupta, S. Pandit, P. Ahmed, Review on herbosomes, a new arena for drug delivery, J Pharmacogn Phytochem, 5(2016):104.
- G.L. Ellman, K. Courtney, V. Andres, R. Featerstone, A new and rapid colorimetric determination of AChE activity, Biochem Pharmacol, 7(1961):88.
- S.A. El-Marasy, S.M. El-Shenawy, A.S. El-Khatib, O.A. El-Shabrawy, S.A. Kenawy, Effect of Nigella sativa and wheat germ oils on scopolamine-induced memory impairment in rats, Bull Fac Pharm Cairo Univ, 50(2012):81-88.
- D.A. El-Sherbiny, A.E. Khalifa, A.S. Attia, D. Eldenshary, Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine, Pharmacol Biochem Behav, 76(2003):525.
- T. Finkel, N.J. Holbrook, Oxidants, oxidative stress and the biology of aging, Nature, 408(2000):239.
- F.M. Garlich, K. Balakrishnan, S.K. Shah, M.A. Howland, J. Fong, et al. Prolonged altered mental status and bradycardia following pediatric donepezil ingestion, Clin Toxicol (Phila) 52(2014):291.
- P. Goverdhan, A. Sravanthi, T. Mamatha, Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress, Int J Alzheimers Dis, 2012(2012):974013.
- J.M. Gutierres, F.B. Carvalho, M.R.C. Schetinger, P. Agostinho, P.C. Marisco, et al. Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats, Int J Dev Neurosci, 33(2104):88.
- J.S. Ha, J.M. Kim, S.K. Park, J.Y. Kang, U. Lee, et al. Anti-amyloidogenic properties of an ethyl acetate fraction from Actinidia arguta in Aβ1-42-induced ICR mice, Food Funct, 9(2018):3264.
- S. Haider, S. Tabassum, T. Perveen, Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: A comparative study, Brain Res Bull, 127(2016):234.
- H.J. Heo, M.J. Kim, J.M. Lee, S.J. Choi, H.Y. Cho, et al. Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia, Dement Geriatr Cogn Disord, 17(2004):151.
- Y. Hou, X. Dan, M. Babbar, Y. Wei, S.G. Hasselbalch, et al. Ageing as a risk factor for neurodegenerative disease, Nat Rev Neurol, 15(2019):565.
- L.G. Howes, Cardiovascular effects of drugs used to treat Alzheimer's disease, Drug Saf, 37(2014):391.
- N. Jain, B.P. Gupta, N. Thakur, R. Jain, Phytosome: A novel drug delivery system for herbal medicine, Int J Pharm Sci Drug Res, 2(2010):224.
- K.L. Alikatte, B.R. Akondi, V.G. Yerragunta, V.R. Prabhakar, P. Suresh, Antiamnesic activity of Syzygium cumini against scopolamine-induced spatial memory impairments in rats, Brain Dev, 34(2012):844.
- R. Kaur, S. Mehan, D. Khanna, S. Kalra, J. Austin, Ameliorative treatment with ellagic acid in scopolamine induced Alzheimer's type memory and cognitive dysfunctions in rats, Austin J Clin Neurol, 2(2015):1053.
- J.M. Kim, S.K. Park, T.J. Guo, J.Y. Kang, J.S. Ha, et al. Anti-amnesic effect of Dendropanax morbifera via JNK signaling pathway on cognitive dysfunction in high-fat diet-induced diabetic mice, Behav Brain Res, 312(2016):39.
- A.D. Korczyn, V. Vakhapova, The prevention of the dementia epidemic, J Neurol Sci, 257(2007):2.
- S.E. Lakhan, A. Kirchgessner, M. Hofer, Inflammatory mechanisms in ischemic stroke: therapeutic approaches, J Transl Med, 7(2009):1.
- G.Y. Lee, C. Lee, G.H. Park, J.H. Jang, Amelioration of scopolamine-induced learning and memory impairment by ÃÃÂ??±-pinene in C57BL/6 mice, Evid Based Complement Alternat Med, 2017(2017):4926815.
- I. Misane, S.O. Ogren, Selective 5-HT(1A) antagonists WAY 100635 and NAD299 attenuate the impairment of passive avoidance caused by scopolamine in the rat, Neuropsychopharmacology, 28(2003):253.
- S.V. More, H. Kumar, D.Y. Cho, Y.S. Yun, D.K. Choi, Toxin-induced experimental models of learning and memory impairment, Int J Mol Sci, 17(2016):1447.
- S.R. Naik, V.W. Pilgaonkar, V.S. Panda, Evaluation of antioxidant activity of Ginkgo biloba phytosomes in rat brain, Phytotherapy Res Int J, 20(2006):1013.
- T.P. Ng, P.C. Chiam, T. Lee, H.C. Chua, L. Lim, et al. Curry consumption and cognitive function in the elderly, Am J Epidemiol, 164(2006):898.
- M. Nishikimi, N.A. Rao, K. Yagi, The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun, 46(1972):849.
- J.H. Oh, B.J. Choi, M.S. Chang, S.K. Park, Nelumbo nucifera semen extract improves memory in rats with scopolamine-induced amnesia through the induction of choline acetyltransferase expression, Neurosci Lett, 461(2009):41.
- H. Ohkawa, N. Ohishi, K. Yagi, Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction, Anal Biochem, 95(1979):351.
- S.K. Richetti, M. Blank, K.M. Capiotti, A.L. Piato, M.R. Bogo, et al. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish, Behav Brain Res, 217(2017):10.
- S. Schaffer, H. Asseburg, S. Kuntz, W.E. Muller, G.P. Eckert, Effects of polyphenols on brain ageing and Alzheimerâ??s disease: Focus on mitochondria, Mol Neurobiol, 46(2012):161.
- R. Schliebs, T. Arendt, The cholinergic system in aging and neuronal degeneration, Behav Brain Res, 221(2011):555.
- K. Sharma, Cholinesterase inhibitors as Alzheimer's therapeutics (review), Mol Med Rep, 20(2019):1479.
- V.K. Sharma, T.G. Singh, N. Garg, S. Dhiman, S. Gupta, et al. Dysbiosis and Alzheimer's disease: A role for chronic stress? Biomolecules, 11(2021):678.
- C. Tapia-Rojas, A. Schuller, C.B. Lindsay, R.C. Ureta, C. Mejias-Reyes, et al. Andrographolide activates the canonical Wnt signalling pathway by a mechanism that implicates the non-ATP competitive inhibition of GSK-3β: Autoregulation of GSK-3β in vivo, Biochem J, 466(2015):415.
- C.V. Vorhees, M.T. Williams, Morris water maze: Procedures for assessing spatial and related forms of learning and memory, Nat Protoc, 1(2006):848.
- Q. Wang, L.H. Sun, W. Jia, X.M. Liu, H.X. Dang, et al. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice, Phytother Res, 24(2010):1748.
- T. Wang, B. Liu, W. Zhang, B. Wilson, J.S. Hong, Andrographolide reduces inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuronglia cultures by inhibiting microglial activation, J Pharmacol Exp Ther, 308(2004):975.
- S.J. Zhang, D. Luo, L. Li, R.R. Tan, Q.Q. Xu, et al. Ethyl acetate extract components of Bushen-Yizhi Formula provides neuroprotection against scopolamine-induced cognitive impairment, Sci Rep, 7(2017):9824.
Copyright: © 2024 Kottapalli Vinay Kumar, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.