Research - Journal of Drug and Alcohol Research ( 2024) Volume 13, Issue 4
Anticancer Effect against Pancreatic Cell Line and Leukemia Cell Line Study of Scoparia Dulcis
Shaik Harun Rasheed, S. Parthyusha*, K.H. Usha Devi, Ravi Kumar Vakkalagadda, K. Sumalatha, Ch. Suresh and P. Bharghava Bhushan Rao2Department of Pharmacy, Bhaskar Pharmacy College, India
3Department of Pharmacy, AM Reddy Memorial College of Pharmacy, India
S. Parthyusha, School of Pharmacy, Guru Nanak Institutions Technical Campus, India, Email: pharmacy.prathyuss@gmail.com
Received: 01-Apr-2024, Manuscript No. JDAR-24-132214 ; Editor assigned: 03-Apr-2024, Pre QC No. JDAR-24-132214 (PQ); Reviewed: 17-Apr-2024, QC No. JDAR-24-132214; Revised: 22-Apr-2024, Manuscript No. JDAR-24-132214 (R); Published: 29-Apr-2024, DOI: 10.4303/JDAR/236294
Abstract
Cancer is a pathological state characterised by the uncontrolled proliferation and dissemination of abnormal cells originating from the body’s own tissues. Evaluating in-vitro cytotoxicity has become a widely used screening method for determining whether natural sources have anticancer potential. Utilising 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide, the MTT test was utilised to assess the antitumour efficaciousness of a hydroalcoholic extract obtained from Salvia dulcis L. Preliminary phytochemical analysis was also performed. To evaluate cell viability and inhibition, the MTT Assay was utilised. Cell viability decreased and growth inhibition increased in a concentrationdependent manner in the hydroalcoholic extract of Scoparia dulcis L. Additionally, it induced changes in cell morphology. S. dulcis IC50 values on Pancreatic and leukaemia cell lines were 104.56 g/ml and 73.51 g/ml, respectively, during a 24 hour incubation period, concentration range of 50 g to 150 g by MTT test. Doxorubicin was utilised as a standard control in the study. According to the findings, the hydroalcoholic extract of S. dulcis displays substantial anti-cancer effect on Pancreatic and leukaemia cell lines.
Keywords
Scoparia dulcis; In-vitro cytotoxicity; Soxhlet extraction; Cytotoxicity
Introduction
The most prevalent and fatal illness nowadays is cancer. The majority of currently used cancer medicines have side effects, and not all cancers respond to treatment in the same way. As a result, novel approaches or chemicals are required. The use of plant-based medications as the main source of chemotherapy treatments has good potential. Additionally, the advantage of third world nations still use herbal items as their main source of health treatment. Accordingly, examining for antitumor chemicals can be done by using plants as herbal remedies Nowadays, you may find Scoparia dulcis L., a small plant of the Plantaginaceae family, as a scattered herb or subshrub in most tropical regions of the world. S. dulcis is employed in traditional medicine as well as a therapeutic agent for the management of gastrointestinal ailments, diabetes, hypertension, and other related conditions. Pharmacological research has shown that the hydroalcoholic extracts of this plant have several therapeutic properties, such as being effective against diabetes, inflammation, fever, microbes, tumours, liver damage, and pain [1-8].
Materials and Methods
Maintenance of cell lines
Acquired from NCCS in Pune, India, the MIA PA CA cell line is a human pancreatic adenocarcinoma. High-glucose DMEM medium was utilized to cultivate the cells, in which 10% FBS and 1% antibiotic-antimycotic solution were added as supplements. At a temperature of 37°C, the culture was maintained in a CO2 incubator with an atmosphere containing 5% CO2 and 18%-20% O2. Every 2 days, the cells were sub cultured. The current investigation made use of Passage 38.
Acquired from NCCS in Pune, India, the THP-1 cell line is a human acute leukemia cell line. High-glucose DMEM medium was utilized to cultivate the cells, in which 10% FBS and 1% antibiotic-antimycotic solution were added as supplements. At a temperature of 37°C, the culture was maintained in a CO2 incubator with an atmosphere containing 5% CO2 and 18%-20% O2. Every 2 days, the cells were sub cultured. The current investigation made use of Passage 38.
Plant material
S. dulcis (Plantaginaceae) was acquired from Sri Venkateswara University in Tirupati, Andhra Pradesh, following verification. These plants are maintained in the Guru Nanak Institutions Technical Campus herbarium, which is part of the School of Pharmacy (Figure 1).

Figure 1: Scoparia dulcis L.
Preparation of extract
The drug substance is placed on a filter paper shaped like a thimble, and then stored in a glass thimble for preservation. The thimble is equipped with both a syphon tube and an inlet tube. At the top, the cylinder encloses the condensed water. The complete assembly is placed into the flask’s neck, which has a circular bottom and a hydroalcoholic solution (70:30) inside of it. The flask should be heated using either a water bath or a heating mantle. The vapours from the solvent travel up the inlet tube and condense on the thimble as they pass into the condenser. The condensed solvent comes into contact with the drug placed in the thimble and dissolves. The solution then reaches the top end of the syphon tube. This method ensures a consistent flow of solvent vapours is maintained in the thimble, allowing the dissolved drug to return to the flask. Finally, the heating process is halted, and the solution in the flask is carefully filtered in order to collect the desired extract (Figure 2).

Figure 2: Soxhlet extraction
Preliminary phytochemical screening
In order to identify multiple active ingredients, a qualitative chemical screening was conducted on the hydroalcoholic extract of S. dulcis. This involved performing various chemical tests [9-12].
MTT assay: To assess the cytotoxicity of a substance on 2 different cell lines. The sample details as shown in Table 1.
Table 1: Specifics of the study samples received
| S. No. | Sample Name/Code | Concentrations (µg/ml) | Cell line |
|---|---|---|---|
| 1 | SD | 5 (12.5,25,50,100 and 200) | MIA PA CA 2 and THP-1 |
The MTT test is a widely used colorimetric technique for evaluating cell growth and cytotoxicity. The function of this is to monitor the formation of formazan crystals through the reduction of MTT, which is a water-soluble tetrazolium dye with a yellow Colour. Liver cells generate mitochondrial lactate dehydrogenase, which converts MTT into insoluble formazan crystals. These crystals become purple when dissolved in an appropriate solvent. The number of living cells is directly proportional to the intensity of this color, which may be measured using a spectrophotometer at 570 nm.
Materials:
1. Cell lines: MIA PA CA 2-Human pancreatic adenocarcinoma cell line (From NCCS, Pune)
2. Cell lines: THP-1-Human acute leukemia cell line (From NCCS, Pune)
3. Cell culture medium: RPMI-1640 medium (Cat No: A10491, Gibco, Invitrogen)
4. Cell culture medium: DMEM high glucose medium-(#12430, Gibco, Invitrogen)
5. Adjustable multichannel pipettes and a pipettor (Benchtop, USA)
6. Fetal Bovine Serum (#RM10432, Himedia)
7. Antibiotic-Antimycotic solution (100x) (Cat No: 15240096, Gibco, Invitrogen)
8. MTT Reagent (5 mg/ml) (#4060 Himedia)
9. DMSO (#PHR1309, Sigma)
10. Doxorubicin (#D1515, Sigma)
11. DMSO (#TL1006, Himedia)
12. 96-well plate for culturing the cells (From Corning, USA)
13. T25 flask (#12556009, Biolite-Thermo)
14. 50 ml centrifuge tubes (#546043 TORSON)
15. 1.5 ml centrifuge tubes (TORSON)
16. 10 ml serological pipettes (TORSON)
17. 10 ul to 1000 ul tips (TORSON)
Equipment:
1. Centrifuge (Remi: R-8C).
2. Pipettes: 2 μl-10 μl, 10 μl-100 μl, and 100 μl-1000 μl.
3. Inverted biological microscope (CKX415F, Olympus, Japan)
4. 37°C incubator with 5% CO2 humidified atmosphere (Healforce, China)
5. Microplate reader with 96 wells (ELX-800, BioTek, USA)
Assay controls:
i. Medium control refers to a control group in an experiment that does not include any cells.
ii. A negative control is a medium that contains cells but does not contain the chemical or substance being tested.
iii. The positive control comprises a cell-containing medium supplemented with a 1 uM/ml concentration of doxorubicin.
Steps followed: For MIA PA CA 2 cells
1. In the absence of the test agent, add 200 μl of a cell suspension containing 20,000 cells per well to each well of a 96-well plate. Allow an estimated 24 hours for the proliferation of the cells.
2. Add the test agent’s specified concentrations as shown in the Excel sheet’s findings section.
3. Place the plate in a controlled environment with a temperature of 37°C and a 5% concentration of carbon dioxide for a duration of 24 hours.
4. Remove the plates from the incubator after incubation. Discard the wasted medium and proceed to add MTT reagent to the entire volume. To achieve 0.5 mg/mL, adjust the concentration.
5. To prevent the plate from being exposed to light, it is advisable to wrap it with aluminium foil.
6. After 3 hours, return the dishes to the incubator and allow them to rest. (Note: Different cell lines require varying times for incubation. When doing comparisons within a single experiment, the incubation period should remain consistent).
7. Subsequent to eliminating the MTT reagent, introduce 100 μl of DMSO solubilization solution.
8. Gyratory shaking with gentle motion will promote dissolution. Occasionally, it may be necessary to perform pipetting movements in both upward and downward directions in order to fully dissolve the MTT formazan crystals in thick cultures.
9. At reference wavelengths of 570 nm and 630 nm, the absorbance should be measured with a spectrophotometer or an ELISA reader.
10. The formula is employed to determine the percentage of cell viability
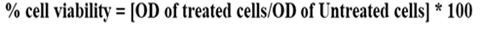
11. Y=Mx+C, a linear regression equation, was used to calculate the IC50 value. In this instance, Y had a value of 50, and the viability graph provided the values for M and C.
Steps followed: For THP-1 cells:
1. In the absence of the test agent, add 200 μl of a cell suspension containing 20,000 cells per well to each well of a 96-well plate. Allow an estimated 24 hours for the proliferation of the cells.
2. Add the test agent’s specified concentrations as shown in the Excel sheet’s findings section.
3. Place the plate in a controlled environment with a temperature of 37°C and a 5% concentration of carbon dioxide for a duration of 24 hours.
4. Remove the plates from the incubator after incubation. Discard the wasted medium and proceed to add MTT reagent to the entire volume. To achieve 0.5 mg/mL, adjust the concentration.
5. To prevent the plate from being exposed to light, it is advisable to wrap it with aluminium foil.
6. After 3 hours, return the dishes to the incubator and allow them to rest. (Note: Different cell lines require varying times for incubation. When doing comparisons within a single experiment, the incubation period should remain consistent).
7. Subsequent to eliminating the MTT reagent, introduce 100 μl of DMSO solubilization solution.
8. Gyratory shaking with gentle motion will promote dissolution. Occasionally, it may be necessary to perform pipetting movements in both upward and downward directions in order to fully dissolve the MTT formazan crystals in thick cultures.
9. At reference wavelengths of 570 nm and 630 nm, the absorbance should be measured with a spectrophotometer or an ELISA reader.
10. The formula is employed to determine the percentage of cell viability
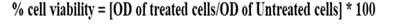
11. Y=Mx+C, a linear regression equation, was used to calculate the IC50 value. In this instance, Y had a value of 50, and the viability graph provided the values for M and C.
The concentrations utilized for the study: In this investigation, we evaluated the test chemical’s cytotoxic effects on the MIA PA CA 2 and THP-1 cell lines. The chemical concentrations employed for cell therapy were as shown in Table 2.
Table 2: Specifics of the drug treatments administered to the cell lines utilized in the study
| S. No. | Test | Cell Line | Concentration treated to cells |
|---|---|---|---|
| 1 | Untreated | MIA PA CA 2 and THP-1 | No treatment |
| 2 | Std. Control | MIA PA CA 2 and THP-1 | 1 µM/ml |
| 3 | Blank | - | Only media without cells |
| 4 | SD2 | MIA PA CA 2 and THP-1 | 12.5 µg/ml, 25 µg/ml, 50 µg/ml,100 µg/ml and 200 µg/ml |
Results
Scoparia dulcis
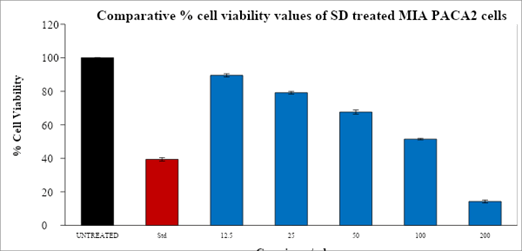
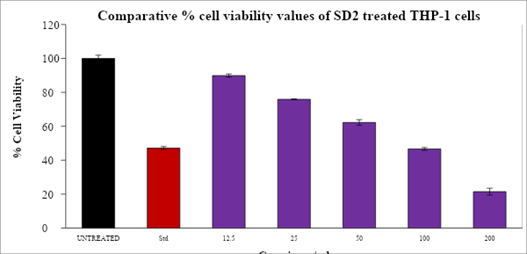
After a 24-hour treatment period, the Table 3 displayed the % cell viability values and IC50 values of the supplied test drug against the Human pancreatic cancer (MIA PA CA 2) cell line and Human acute leukemia cell line (THP-1) and was depicted in bar graphs as shown below Figure 3.
Table 3: MTT assay-summary-cell viability values
| Drug conc. (µg/ml) | MIA PA CA2 | THP-1 |
|---|---|---|
| Untreated | 100 | 100 |
| Dox-1 µM | 39.37 | 47.14 |
| SD-12.5 µg | 89.51 | 89.89 |
| SD-25 µg | 79.13 | 75.92 |
| SD-50 µg | 67.6 | 62.25 |
| SD-100 µg | 51.46 | 46.62 |
| SD-200 µg | 14.26 | 21.45 |
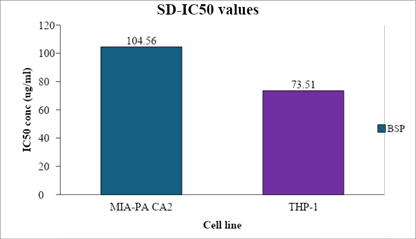
| IC50 Conc (µg/ml) | 104.56 | 73.51 |
Figure 3: An overlay bar graph depicted the proportion of viable cells of MIA PA CA 2 cells treated with varied doses of SD2 after a 24-hour incubation period
After an incubation period of 24 hours, the superimposed bar graph illustrates the proportion of viable THP-1 cells that were exposed to various concentrations of SD2 (Figure 4).
Figure 4: The proportion of viable THP-1 cells that were exposed to various concentrations of SD2
Figure 5 depicted the IC50 values of SD against the MIA PA CA 2 and THP-1.
Figure 5: Comparative Bar graph depicted the IC50 values of SD against the MIA PA CA 2 and THP-1 cells after the incubation period of 24 hours
Discussion
Cytotoxicity study of the SD against MIA PA CA 2 and THP-1 cell line
With an IC50 value of 104.56 ug/ml, the test chemical SD2 showed moderate toxicity against MIA PA CA2 and THP-1 cells, according to statistical results from the cytotoxicity research utilising the MTT assay. Furthermore, after a 24-hour incubation period, the test compound showed effective toxicity with an IC50 value of 73.51 ug/ml. The study included doxorubicin as the standard control [13-17].
Microscopic examination of drug-treated pictures of the THP-1 cell line and MIA PA CA 2 obtained at a 20x magnification.
Conclusion
S. dulcis have effective potential capacity against human pancreatic cancer cells with IC50 value 104.56 μg/ml and effective toxicity on human acute leukemia cells with IC50 value of 73.51 μg/ml. S. dulcis have more cytotoxic activity against leukemia cell when compared with Pancreatic cancer cells.
Acknowledgement
None.
Conflict Of Interest
The authors declared that they have no conflict of interest.
References
- J. Ferlay, M. Ervik, F. Lam, M. Colombet, L. Mery, et al. Global cancer observatory: Cancer today, Lyon: IARC, 2020.
- C. de Martel, D. Georges, F. Bray, J. Ferlay, G.M. Clifford, Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis, Lancet Glob Health, 8(2020):e180-e190.
- Assessing national capacity for the prevention and control of non-communicable diseases: Report of the 2019 global survey, Geneva: WHO, 2020.
- The plant list: A working list of all plant species, 2016.
- USDA, NRCS, Scoparia dulcis, The Plants Database, 2015.
- R. Jain, M. Singh, Factors affecting goatweed (Scoparia dulcis) seed germination, Weed Sci, 37(1989):766-70.
- Scoparia dulcis, Germplasm Resources Information Network, Agricultural Research Service, 2018.
- S. Majumder, M.M. Rahman, S.K. Bhadra, Micropropagation of Scoparia dulcis Linn. through induction of indirect organogenesis, J Mol Biol Biotechnol, 19(2011):11-17.
- L. Pari, M. Latha, Protective role of Scoparia dulcis plant extract on brain antioxidant status and lipidperoxidation in STZ diabetic male wistar rats, BMC Complement Altern Med, 4(2004):16.
- S.M.D.F. Freire, L.M.B. Torres, C. Souccar, A.J. Lapa, Sympathomimetic effects of Scoparia dulcis L. and catecholamines isolated from plant extracts, J Pharm Pharmacol, 48(1996):624-628.
- M. Ahmed, H.A. Shikha, S.K. Sadhu, M.T. Rahman, B.K. Datta, Analgesic, diuretic, and anti-inflammatory principle from Scoparia dulcis, Die Pharmazie, 56(2001):657-660.
- Phan, M. Giang, Chemical and biological evaluation on scopadulane-type diterpenoids from Scoparia dulcis of Vietnamese origin, Chem Pharm Bull, 54(2006):546-549.
- D. Gerlier, N. Thomasset, Use of MTT colorimetric assay to measure cell activation, J Immunol Methods, 94(1986):57-63.
- M.C. Alley, D.A. Scudiero, A. Monks, M.L. Hursey, M.J. Czerwinski, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay, Cancer Res, 48(1988):589-601.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, J Immunol Methods, 65(1983):55-63.
- M.C. Alley, D.A. Scudiere, A. Monks, M. Czerwinski, R. Shoemaker, Validation of an automated Microculture Tetrazolium Assay (MTA) to assess growth and drug sensitivity of human tumor cell lines, Proc Am Assoc Cancer Res, 27(1986):389.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, J Immunol Methods, 65(1983):55-63.
Copyright: © 2024 Shaik Harun Rasheed, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.