Research Article - Journal of Drug and Alcohol Research ( 2021) Volume 10, Issue 10
Characterization of Gums and Their Influence on the Phenomenon of Drug Release
Kameswara Rao Sankula* and Seetha Devi AKameswara Rao Sankula, Department of Pharmacy, Acharya Nagarjuna University, India, Email: Sankula123@yahoo.com
Received: 23-Nov-2021;Accepted Date: Dec 07, 2021; Published: 14-Dec-2021
Abstract
The main concern of the present investigation is to provide new sustained release excipient which, when incorporated into a final product, produces controlled release of active ingredient over an extended period of 12hrs or more when the dosage form is exposed to G.I fluids in gastric environment. Many naturally available polymers were being investigated for their applications in the design of sustained drug delivery systems. The natural polymers which were characterized in the present research work have showed good flow properties and suitable physico chemical properties like low moisture content which enables low chance of microbial growth, high swelling index indicates high swellability and goodwater retention capacity there by enhanced permeation of drug for longer periods of time. The gums exhibiting non-Newtonian plastic flow, this indicates the gelling behavior and confirms their suitability in the development of controlled release delivery systems.
FTIR and DSC studies were carried out for characterization of gums and results indicated that there was no change in the absorption spectra. The bacterial and fungal counts were within limits and pathogenic microorganisms were absent and acute toxicity studies indicated that these gumswere safe to use.
Keywords
Physico-chemical Properties; Bhara gum; Grewia gum; Mucuna Gum; Rheological Properties
Introduction
Nature has provided a wide variety of materials to help mankind and improves the health of all living things either directly or indirectly. Excipients are the one which do not interfere with physical, chemical and pharmacological effects of the active ingredients but they assist in getting desired pharmacological effect from the dosage forms and helps in getting safe and effective dosage form suitable for administration to patients. The excipients are broadly classified in to three types, based on their nature of origin as natural, semi synthetic and synthetic.
These natural excipients have advantages over synthetic ones since they are chemically inert, nontoxic, less expensive, biodegradable and widely available. Natural excipients obtained from plants have diverse applications in drug delivery and they play different roles in dosage forms as binders, disintegrants, emulsifying agents and suspending agents. Now a day’s extensive studies are being conducted for finding the applicability of these natural excipients in the design of novel drug delivery systems [1-6].
Advantages of natural gums in pharmaceutical science
Naturally available biodegradable polymers are produced by all living organisms. All these plant materials are chemically carbohydrates composed of repeating sugar (monosaccharide’s) units. Hence, they are non-toxic. Production cost will be always less with that of natural polymers when compared to synthetic polymers. Environmental friendly processing, these natural gums were easily collected from different sources in various seasons in large quantities because their production and processing cost is very less.
Local availability is more in developing countries like India government promotes the production of plants like guar gum and tragacanth because they have wide applications in various industries. Better patient tolerance as well as public acceptance these natural polymers have less side effects when compared to synthetic one, for example, povidone. Most of these gums were obtained from edible sources.
Disadvantages of natural gums in pharmaceutical science
Originally these gums was carbohydrates and they contain 10% moisture content, during production they are exposed to external environmental conditions so there is a more risk of microbial contamination. It can be prevented by the addition of suitable preservatives. Batch to batch variation is very common problem with natural polymers because their production depends on seasonal factors. Uncontrolled rate of hydration is another drawback; it is due to differences in the collection of natural polymers at different times, as well as differences in region, species, and climate conditions resulting changes in the percentage of chemical constituents.
Generally these gums show high viscosity when they come in contact with water due to complex formation of gums. But it has been found that after storage there is reduction in viscosity.
Materials and Methods
Bhara gum
Taxonomic classification of Bhara gum (www.itis.gov.in)
Kingdom: Plantae
Subkingdom: Viridiplantae
Class: Magnoliopsida
Order: Myrtales
Family: Combretaceae
Genus: Terminalia
Species: Terminalia bellirica
Gum Bhara is a yellowish natural gum of plant Terminalia bellerica roxb. (Bhara) belongs to family Combretaceae. It has been mainly used as a demulcent and purgative. It is also used as an emulsion in cosmetic industries. These wide applications of Bhara gum propose their hydrophilic nature, and compatibility with the physiologic environment. Hence an attempt was made in this current study to investigate the ability of Bhara gum in Gastro retentive drug delivery system (GRDDS).
Grewia gum
Taxonomic classification of Grewia gum
Kingdom: Plantae
Subkingdom: Tracheophyta
Class: Angiosperms
Order: Eudicots
Family: Malvaceae
Genus: Grewia
Species: Mollis
Grewia gum is obtained from the inner bark of the edible plant Grewia mollis (Malvaceae). The gum has been investigated in terms of its use as binding, compressional, drug release matrix and film forming, bioadhesive, suspending and coating agent. The gum was reported to have excellent binding performance which is superior to that of maize starch and gum arabic, and release sustaining behavior which is better than HPMC, CMC and gum arabic.
Mucuna gum
Taxonomic classification of Mucuna gum
Kingdom: Plantae
Subkingdom: Tracheophyta
Class: Angiosperms
Order: Fabales
Family: Fabaceae
Genus: Mucuna
Species: Pruriens
The polymer proposed in this work (mucuna gum) is a biodegradable polymer. It was obtained from the plant Mucuna flagillepes (Fabaceae). In the pharmaceutical arena, mucuna gum has been shown to be a good suspending agent and stabilizer in suspensions and emulsions, a good binder in compressed tablets. The film properties of mucuna gum have been studied as well.
Physico chemical properties
In the present study the gums were obtained from Yarrow Chem. Products, Mumbai and they were evaluated for following tests like solubility, phytochemical tests, powder properties, Mucunasture content, pH, swelling index, volatile acidity and rheological properties [7-11].
Organoleptic evaluation and solubility behavior
Organoleptic properties like color (light, strong, white or brown), odor (odorless, aromatic, pungent or strong) was evaluated, and presence of adulterants can be estimated by solubility studies. Solubility studies were done in water and different solvents like methanol, ethanol, acetone and ether.
Determination of purity and identification tests for gums
Identification tests for obtained gums were carried out by using RGI and RGII reagents as recommended by Association of Official Analytical Chemists (AOAC) (1984). As per the specifications of Food and Agriculture Organization (FAO) (1991) (FAO, 1991) the gums were tested for swelling by ethanol solution and color reaction with conc. HCl, 5N NaOH, aqueous methylene blue and concentrated sulphuric acid.
Determination of powder properties
Bulk density: The bulk density of a powder is the ratio of the mass of an untapped powder to its bulk volume. Required quantity of dry powder (W) was weighed and transferred into 100 ml measuring cylinder. Powder was carefully leveled without compaction and initial volume (Vb) was noted. Bulk density was calculated by the following Eq. 3.1.
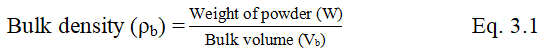
Tapped density (USP 2006)
The required quantity of powder was weighed and transferred to graduate measuring cylinder
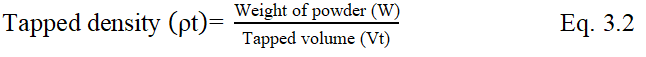
Bulkiness
Reciprocal of bulk density gives the bulkiness. Bulkiness was calculated by the following Eq. 3.3
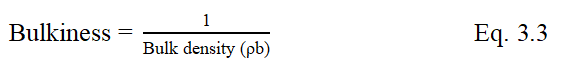
Compressibility Index (I) and Hausner ratio
Compressibility index or Carr’s index and Hausner ratio are used as indicators for flowability and compressibility of the powder. They were calculated by the following Eq. 3.4 and 3.5 respectively
Flow properties
Flow properties of powders were determined by the angle of repose technique. Fixed funnel method was used to determine the angle of repose. The gum powder was carefully poured through the powder funnel until the apex of the conical pile just touched the tip of the funnel. The radius (r) of the base of the pile was determined and the angle of repose (θ) was calculated by the following Eq. 3.6.

Determination of moisture content
Moisture content was determined by using Karl-Fischer auto titrator M/s. Systronics, Model No. 349. Accurately 1 g of gum powder was weighed and transferred into dried titrating flask.
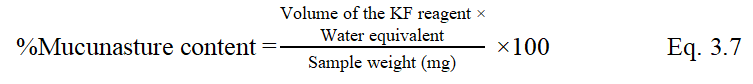
Determination of pH value
The pH of 1% w/v aqueous solution of gums was determined by using pH meter (Systronics, Model No. 361). 2.7 Determination of swelling index and water retention capacity
The swelling indices of gums were determined by placing one gram of powder in a measuring cylinder. The initial volume (Xo) of the powder in a measuring cylinder was noted. The volume was made up to 100 ml mark with water at room temperature. The cylinder was stoppered and shaken gently and set aside for 24 hours. The volume (Xt) occupied by the gum sediment was noted after 24 hours. The swelling index of the gum was calculated by the fallowing Eq. 3.8
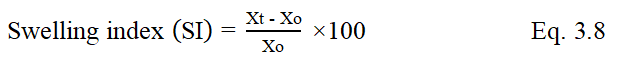
Determination of volatile acidity
About 1 gm of the gum was accurately weighed, transferred to a 800 ml long necked flask, 100 ml of water and 5 ml of orthophosphoric acid were added and allowed to stand until the gum was completely swollen (approximately 24 hours). Then, it was boiled for 2 hours under a reflux condenser steam distilled until 600 ml of the distillate was obtained.
The distillate was titrated with 0.1 N sodium hydroxide using phenolphthalein as indicator. The procedure was repeated omitting the sample. The difference between the two titrations represented the amount of alkali required to neutralize the volatile acid.
Each ml of 0.1 N NaOH=0.006005 g of C2H4O2
Fourier Transforms Infrared Spectroscopy (FTIR)
Fourier transform infrared (FTIR) spectroscopy studies provide the information of chemical functional groups of the sample. The FTIR spectral analysis of gum powders was done by using press pellet technique using potassium bromide (KBR). The FTIR spectrum was recorded by using Perkin-Elmer 841IR spectrometer in the region between 4,000 and 400 cm-1.
Differential Scanning Calorimetry (DSC)
Thermal properties of gum powders were characterized by using differential scanning calorimeter (Shimadzu DSC- 50) at a heating rate of 10°C/min from 30 to 300°C in nitrogen atmosphere (30 ml/min).
Determination of rheological properties of gums
The rheological properties of gums were evaluated using Brookfield cone and plate viscometer (model LV DV-III+Rheometer). Bhara gum, Grewia gum and Mucuna gum concentrations of 5, 10, 15% (w/v) with distilled water were analyzed for its viscosity, shear stress, rate of shear at various speeds and also tested for its thixotropic phenomena at 25°C using CP 52 spindle and Rheocalc software of the instrument.
Microbiological studies on gums
Microbial studies were conducted as per Indian Pharmacopoeia for aerobic, fungal and pathogenic microorganisms. To estimate the number of viable bacteria and fungi, plate count method was used. Liquefied agar medium was used for bacteria and potato agar medium was used for fungi. 1 g/10 ml of gum solution was placed into each liquefied sterilized agar medium and the medium was poured into petri plate and allowed to solidify. The gum sample was estimated for the presence of pathogenic microorganisms by using specific individual media like mannitol salt agar medium (Staphalococcus aureus), cetrimide agar (Pseudomonas aeruginosa), Mac Conkey agar medium (Escherichia coli) and deoxycholate citrate agar medium (Salmonella). Culture mediums prepared according to the procedure given in the IP.
Results and Discussion
Organoleptic evaluation and solubility behavior of gums
Gum powders were subjected to sensory evaluation to determine the organoleptic characters like colour and odour. The organoleptic characters and solubility behavior are summarized in Table 1.
| Parameter | Observation | ||
|---|---|---|---|
| Bhara gum | Grewia gum | Mucuna gum | |
| Colour | Yellow to dark brown | Clear to pale yellow | Yellowish to white |
| Odour | Odourless | Odourless | Odourless |
| Solubility in water | Soluble, forming colorless mucilage | swellable in presence of water | Soluble, forming mucilage |
| Solubility in solvents (chloroform and methanol) | Soluble | Soluble | Soluble |
Table 1: Organoleptic evaluation and solubility behavior of gums.
Determination of purity and identification tests for gums
The specifications of identification and purity for food additives or formulations established by AOAC and FAO are intended to ensure the safety to all. These specific reactions will help in characterizing natural product. To one gram of gum powders 5 ml of reagent was added approximately and results are shown in Tables 2 and 3. The results indicate the presence of polysaccharide.
| Reagent | Bhara gum | Grewia gum | Mucuna gum |
|---|---|---|---|
| RGI | Positive (slight blue colour was observed) | Positive (slight blue colour was observed) | Positive (slight blue colour was observed) |
| RGII | Positive, swells. Pink colored granular mass was obtained | Positive, swells. Pink colored granular mass was obtained | Positive, swells. Pink colored granular mass was obtained |
Table 2: Identification test for food hydrocolloids as per AOAC, 1984
| Test | Observation | ||
|---|---|---|---|
| Bhara gum | Grewia gum | Mucuna gum | |
| Swelling by ethanol solution | Swelling is observed | Swelling is observed | Swelling is observed |
| Colour reaction with Conc. HCl | Brownish yellow colour is observed | Yellow colour is observed | Yellow colour is observed |
| Colour reaction With 5N NaOH | Brownish yellow colour is observed | Yellow colour is observed | Yellow colour is observed |
| Aqueous methylene blue stain | Stained | Stained | Stained |
| Conc.sulphuric acid | No colour change is observed | No colour change is observed | No colour change is observed |
Table 3: Identification test for gums as per FAO, 1991.
Determination of powder properties
Powders normally flow under the influence of gravity; dense substances are generally less cohesive than lighter ones, as the weight of the particles for a given volume is increased. Hence, differences in densities of various ingredients of formulation may lead to improper mixing and filling during manufacturing of formulation results in weight variation and variations in content uniformity of finished products. Results are shown in Table 4.
| Property | Bhara gum | Grewia gum | Mucuna gum |
|---|---|---|---|
| Bulk density (gm/cc) | 0.632±0.04 | 0.599±0.21 | 0.612±0.37 |
| Tapped density (gm/cc) | 0.702±0.02 | 0.718±0.31 | 0.688±0.68 |
| Bulkiness | 1.58±0.04 | 1.69±0.13 | 1.74±0.36 |
| Compressibility index (%) | 9.82±1.34 | 8.76±0.68 | 10.01±0.6 |
| Hausner’s ratio | 1.02±0.054 | 1.10±0.21 | 1.00±0.62 |
| Angle of repose (º) | 28.20±1.28 | 25.12±0.36 | 26.60±0.45 |
| Moisture content | 14.96±1.12 | 12.56±0.12 | 13.59±0.32 |
| pH | 4.8±0.20 | 4.0 | 4.2 |
| Swelling index (%) | 120±10.00 | 122±8 | 118±6 |
Table 4: Physico-chemical properties of gums.
Determination of moisture content
The Moisture content was found to be 14.96%, 12.56% and 13.59% in Bhara gum, Grewia gum and Mucuna gum respectively. Pharmacopoeial limit for Mucunasture contents of natural gums has been set at ≤15.0. The Mucunasture content of gums was found to be low suggesting their suitability to the formulations containing Mucunasture sensitive drugs. Presence of Mucunasture content influences the tabletting properties and also promotes the growth of microorganisms thereby causing stability problems of the drug. As the Mucunasture content was found to be low in all the three gums, they can be considered suitable for the design of controlled release formulations.
Determination of pH value
The pH of 1% w/v Bhara gum, Grewia gum and Mucuna gum powders were found to be 4.8, 4.0 and 4.2 at room temperature indicating that all three gums were acidic in nature and the results are shown.
Determination of swelling index and water retention capacity
The swelling index of Bhara gum, Grewia gum and Mucuna gum were 120%, 122% and 118%. High value of swelling index revealed the high swelling ability of Bhara gum, Grewia gum and Mucuna gum. The swelling ability of any polysaccharide depends upon its water retention capacity (or) water absorption capacity. The water absorption capacity of Bhara gum, Grewia gum and Mucuna gum were 19 mL/g, 22 mL/g and 18 mL/g. It was reported that swelling of a linear polymer without dissolution is an indication that it is cross linked. The swelling ability of polymers enables it to absorb water and reduce fluidity so these are decided as suitable gums for preparation of controlled release dosage forms. The results are shown in Figures 1-3.

Figure 1: Bhara gum.

Figure 2: Grewia gum.

Figure 3: Mucuna gum.
Determination of volatile acidity
The volatile acidity of Bhara gum, Grewia gum and Mucuna gum were found to be 16.8%, 17.2%, and 15.9%. It was reported that the viscosity of gum is directly proportional to the volatile acidity of gum. All the physico chemical parameters of Bhara gum, Grewia gum and Mucuna gum are summarized and results are given.
Fourier transforms infrared spectroscopy
The principal absorption spectra of Bhara gum at 1648 cm-1 (stretching of carbonyl group absorbance) and 3101 cm-1 (stretching of hydroxyl group of carboxylic acid) were observed. The IR spectrum of Bhara gum was shown in Figure 4. The principal absorption peaks of Grewia gum at 1707 cm-1 (stretching of carboxylic group absorbance) and 3101 cm-1 (stretching carboxylic acid) were observed. The IR spectrum of Grewia gum was shown in Figure 5.
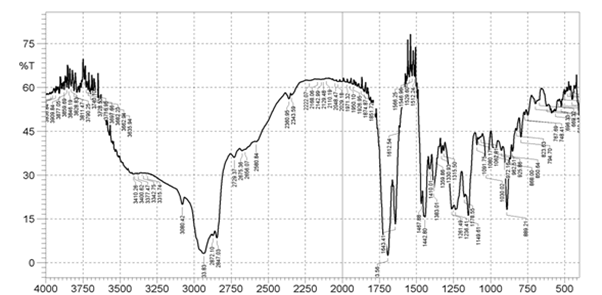
Figure 4: FTIR Spectra of Bhara gum powder.
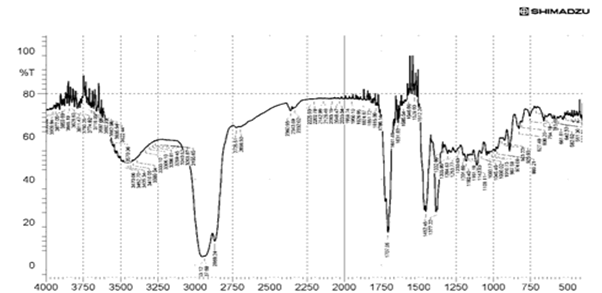
Figure 5: FTIR Spectra of Grewia gum powder
The principal absorption peaks of Mucuna gum at 1149 cm-1 (stretching of alcohol group absorbance) and 3493 cm-1 (stretching of hydroxyl group of carboxylic acid) were observed. The IR spectrum of Mucuna gum was shown in Figure 6. The IR spectral data was given in Table 5.
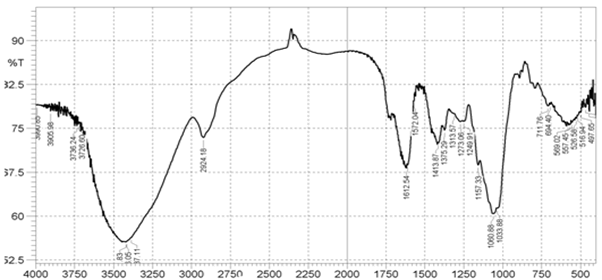
Figure 6: FTIR Spectra of Mucuna gum powder
| Name of the gum | Functional groups | Wave number of gums (cm-1) |
|---|---|---|
| Bhara gum | C-O Phenolic stretch | 1377.22 |
| Ar-H | 1452.45 | |
| C=O stretching | 1707.06 | |
| OH Stretching | 3101.12 | |
| Grewia gum | C-O Alcohol stretch | 1060.88 |
| Ar-C=C | 1612.54 | |
| C-H stretch | 2924.18 | |
| OH Stretching | 3483.00 | |
| Mucuna gum | C-H | 889.21 |
| C-O Alcohol stretch | 1149.61 | |
| C-H bending | 1442.80 | |
| C=O stretch | 1695.18 | |
| OH Stretching | 3493.83 |
Table 5: FTIR spectral data of gums
Differential Scanning Calorimetry (DSC)
A sharp symmetric melting endotherm can indicate crystallinity of the sample, where as broad, asymmetric curve represents the non crystallinity of the sample. The endothermic peak usually indicates the loss of water present in the compound. The DSC thermograms of Bhara, Grewia and Mucuna gums are shown in Figures 7 and 8. Bhara gum exhibited broad endothermic peak at 109°C. Grewia gum exhibited broad endothermic peak at 180.9°C. Mucuna gum exhibited broad endothermic peak at 111°C. Therefore the DSC studies indicated the non-crystalline nature of gums.
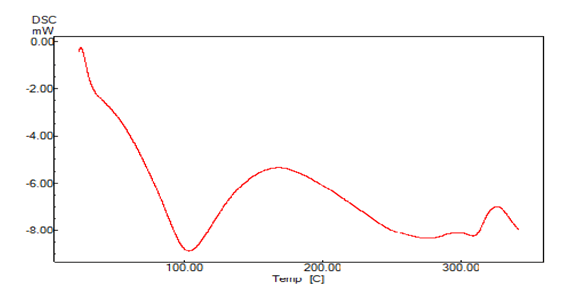
Figure 7: DSC thermogram of Bharagum powder
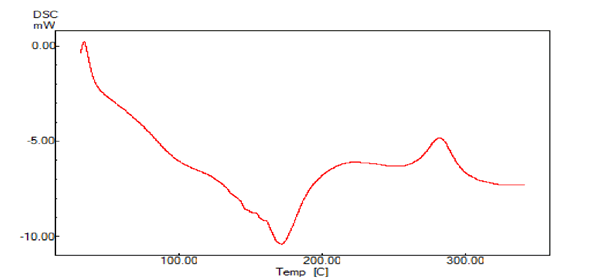
Figure 8: DSC thermogram of Grewia gum powder
Determination of rheological properties of gums
The rheological properties of the gum play major role in the release of drug from the dosage form. It is necessary in particular to the polysaccharide materials to check the viscosity
To determine the rheological properties of Bhara, Grewia and Mucuna gums, different concentrations were prepared and evaluated by using Brookfield cone and plate viscometer (model LV DV-III+Rheometer) and corresponding rheograms are shown in Figures 9-18.
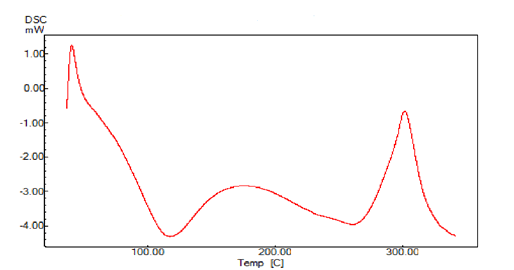
Figure 9: DSC thermogram of Mucuna gum powder
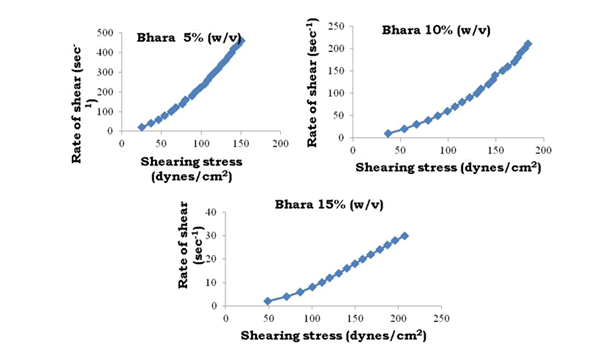
Figure 10: Rheograms of Bhara gum
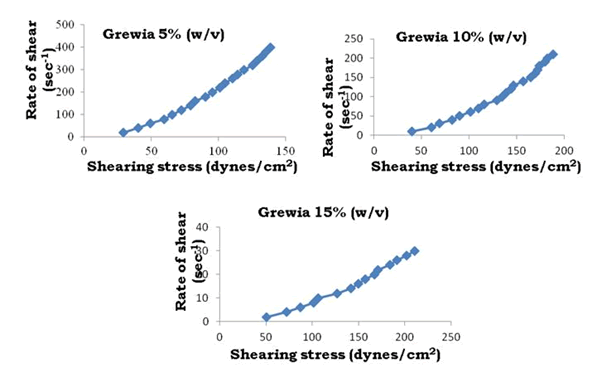
Figure 11: Rheograms of Grewia gum
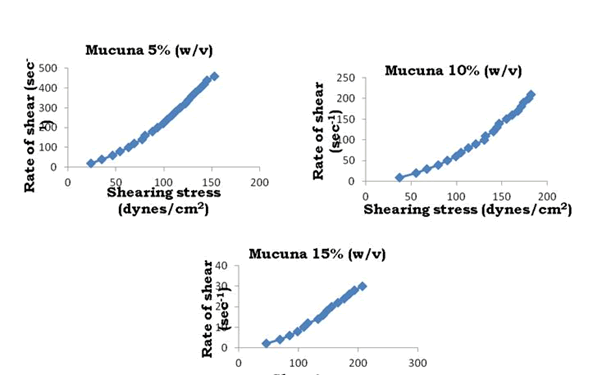
Figure 12: Rheograms of Mucuna gum
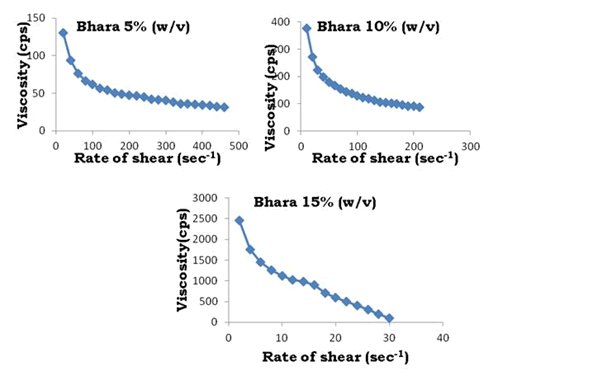
Figure 13: Rate of shear vs. viscosity profiles of Bharagum
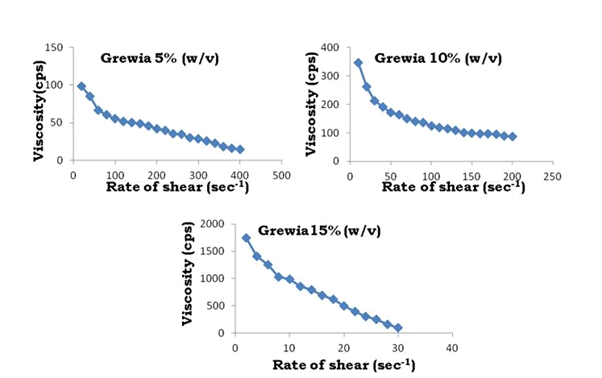
Figure 14: Rate of shear vs. viscosity profiles of Grewia gum
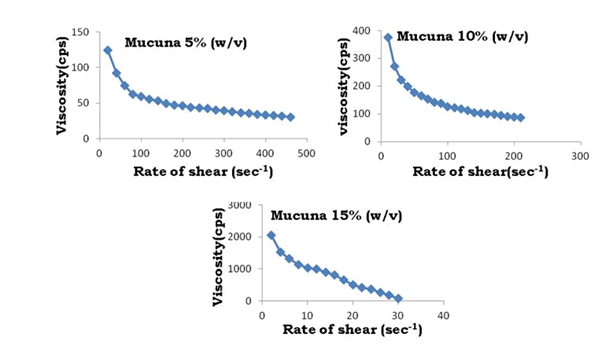
Figure 15: Rate of shear vs. viscosity profiles of Mucuna gum
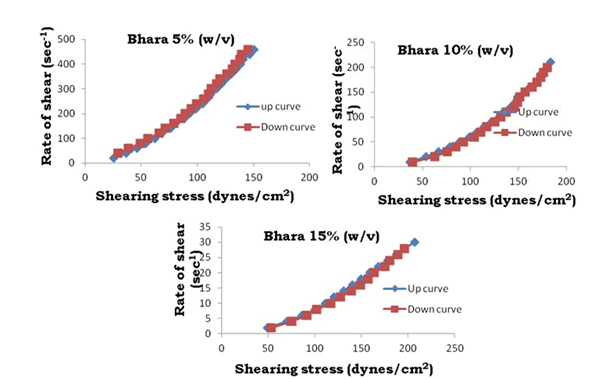
Figure 16: Thixotrophy rheograms for Bhara gum
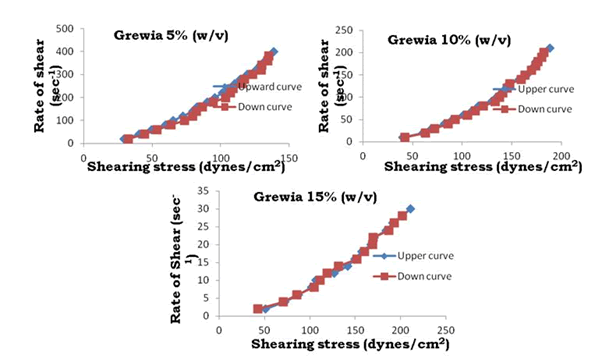
Figure 17: Thixotrophy rheograms for Grewia gum
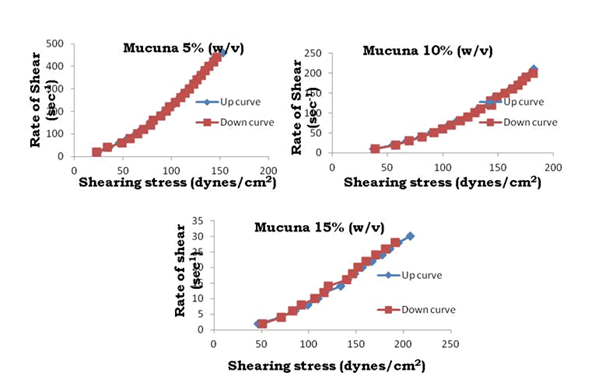
Figure 18: Thixotrophy rheograms for Mucuna gum
The Newtonian equation for viscosity has been modified to characterize non-Newtonian behavior. Some of the widely used equations are summarized below Whereas=shear stress, 0=yield stress, D=shear rate, η=plastic viscosity, k=- consistency index, p=flow index. A power law index of p=1 represents Newtonian flow behavior, p<1 represents shear thinning (pseudoplatic) behavior and p>1 represents shear thickening (dilatants) behavior.

The viscosity of gums was found to be dependent on concentration at the same shear rate. It increases with increase in gum concentration. Rheograms were plotted between shear rate and shear stress and it was observed that there was no difference between the upward and downward curve indicating the absence of thixotropy. The viscosity data was analyzed by using Bingham, Casson and Power law equations and the results are shown in Table 6.
| Bingham | Concentration of Bhara gum (%) | Concentration of Grewia gum (%) | Concentration of Mucuna gum (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 5 | 10 | 15 | 5 | 10 | 15 | |
| η (cps) | 25.5 | 454.6 | 1324 | 24.6 | 429.2 | 1225 | 25.5 | 454.6 | 1324 |
| τ0(dynes/cm2) | 34 | 56 | 64.8 | 39 | 61 | 71.2 | 34 | 56 | 64.8 |
| Cof (%) | 92.5 | 92.5 | 93 | 90.8 | 89.7 | 91.2 | 89.6 | 83.8 | 95.3 |
| Casson | |||||||||
| η (cps) | 15.6 | 248.7 | 677.9 | 18.2 | 242.3 | 677.9 | 15.6 | 248.7 | 677.9 |
| τ0(dynes/cm2) | 13 | 26.2 | 32.7 | 15 | 22.3 | 32.7 | 17 | 29.2 | 35.9 |
| Cof (%) | 96.9 | 95.7 | 95.4 | 90.1 | 97.4 | 94.5 | 90.4 | 96.8 | 92.1 |
| Power Law | |||||||||
| Consistency index, k(cps) | 462.9 | 3419 | 6458 | 450.2 | 3310 | 6125 | 431.2 | 3320 | 6325 |
| Flow index, n | 0.49 | 0.49 | 0.45 | 0.35 | 0.41 | 0.4 | 0.37 | 0.41 | 0.44 |
| Cof (%) | 96 | 93.7 | 91.9 | 92 | 94.7 | 94.9 | 95 | 96.7 | 96.9 |
Table 6: Analysis of rheological properties of Bhara, Grewia and Mucuna gums
Gums in aqueous solution obeyed power law with correlation coefficient values ranging from 91.9 to 96.9. Bhara, Grewia and Mucuna gums hydrates quickly and swells rapidly and form a thick viscous layer around it. This is the most important criterion required for hydrophilic matrix systems.
The gums exhibited non-Newtonian plastic flow which was indication for the gelling behavior and confirmed their suitability in the development of controlled release delivery systems.
Microbiological studies on gums
Microbial studies were carried out for samples immediately after collection and processing of the gums. Results were given in Table 7. After incubation period the total aerobic microorganisms observed were 20, 18 and 25 CFU/g respectively, which was less than the recommended value (NMT 100 CFU/g) as per IP. No growth was observed in the culture media of pathogenic microorganism after inoculation and incubation for specified period. Results proved that the gums did not support bacterial and fungal growth. Gums did not contain any pathogenic microorganisms like E. coli, Pseudomonas aeruginosa, Staphylococcus aureus and Salmonella sp. Microbial quality of gums was satisfactory and met the Indian Pharmacopoeia requirements of microbiological quality of pharmaceutical preparations.
| Parameter | Bhara gum | Grewia gum | Mucuna gum | Specification |
|---|---|---|---|---|
| Total aerobic microbial count (CFU/gm) | 20 | 18 | 25 | Not more than 100 |
| Total fungal count (CFU/gm) | 21 | 14 | 20 | Not more than 100 |
| Pathogens a. S. aureus b. P. aeruginosa c. E. coli d. Salmonella |
a. Absent/g b. Absent/g c. Absent/g d. Absent/g |
a. Shall be absent/g b. Shall be absent/g c. Shall be absent/g d. Shall be absent/g |
||
Table 7: Microbial studies on gums
Conclusion
The gums which were characterized in the present research work were showed good flow properties and suitable physico chemical properties like low Moisture content enables low chance of microbial growth, high swelling index indicates high swellability and good water retention capacity there by enhanced permeation of drug for longer periods of time. The gums exhibiting non-Newtonian plastic flow which is indication for the gelling behavior and confirmed their suitability in the development of controlled release delivery systems.
FTIR and DSC studies were carried out for characterization of gums and results indicated that there was no change in the absorption spectra. The bacterial and fungal counts were within limits and pathogenic microorganisms were absent and acute toxicity studies indicated that these gums were safe to use
References
- A. Y. Kaushik, A. K. Tiwari, Anand Gaur, Role of excipients and polymeric advancements in preparation of floating drug delivery systems , Int J of Pharm Invsgn, 5 (2015), 1–12.
- S. Kumar, S. K. Gupta, Natural polymers, gums and mucilages as excipients in drug delivery, Polim Med, 42 (2012), 191-7.
- N. Iglesias, E. Galbis, L. Romero Azogil, E. Benito, In-Depth study into polymeric materials in low-density gastroretentive formulations, J Pharms, 12 (2020), 636.
- P. Dorozyński, R. Jachowicz, P. Kulinowski, S. Kwieciński, K. Szybiński, The macromolecular polymers for the preparation of hydrodynamically balanced systems--methods of evaluation, Drug Dev Ind Pharm, 30 (2001), 947-57.
- Z. Li, R. Zeng, L. Yang, Development and characterization of pcl electrospun membrane-coated bletilla striata polysaccharide-based gastroretentive drug delivery system, AAPS Pharm Sci Tech, 21 (2020), 66.
- X. Qi, H. Chen, Y. Rui, F. Yang, N. Ma, et al., Floating tablets for controlled release of of loxacin via compression coating of hydroxypropyl cellulose combined with effervescent agent”, Int J Pharm, 489 (2015), 210–7.
- M. S. Amiri, V. Mohammadzadeh, Plant-Based gums and mucilages applications in pharmacology and nanomedicine: A review, Molecules, 26 (2021), 1770.
- A. C. Mendes, E. T. Baran, Palmitoylation of xanthan polysaccharide for self-assembly microcapsule formation and encapsulation of cells in physiological conditions, Sft Mttr, 7 (2011), 9647–9658.
- N. Jindal, Singh, Kinetics and physico-chemical characterization of exopolysaccharides produced by the cyanobacterium Oscillatoria Formosa, World J Microbiol Biotechnol, 27 (2011), 2139–2146.
- S. Parija, M. Misra, A. K. Mohanty, Studies of natural gum adhesive extracts: An overview, J Macromol Sci Polym Rev C, 41 (2001), 175–197.
- H. Moritaka, S. Kimura, H. Fukuba, Rheological properties of matrix particle gellan gum gel: Effects of calcium chloride on the matrix, Food Hydrocoll, 17 (2003), 653–660

