Research Article: Journal of Drug and Alcohol Research (2024) Volume 13, Issue 11
Comorbidity of Alcohol Use Disorder and Neurocysticercosis Aggravated by Dysbiosis
Lourdes de Fatima Ibanez Valdes1 and Foyaca-Sibat Humberto2*2Department of Neurology, Walter Sisulu University, South Africa
Foyaca-Sibat Humberto, Department of Neurology, Walter Sisulu University, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 30-Oct-2024, Manuscript No. JDAR-24-150603; Editor assigned: 01-Nov-2024, Pre QC No. JDAR-24-150603 (PQ); Reviewed: 15-Nov-2024, QC No. JDAR-24-150603; Revised: 20-Nov-2024, Manuscript No. JDAR-24-150603 (R); Published: 27-Nov-2024, DOI: 10.4303/JDAR/236417
Abstract
Background: Neurocysticercosis is a zoonotic and neglected parasitic disorder affecting the cerebral hemispheres, the brainstem, the cerebellum, the spinal cord, the optic nerve and all brain cavities and covering all patients who complain of headaches and epilepsy. Comorbidity among NCC/OSAS has not been study.
Method: We searched the articles published in the medical literature comprehensively, looking for Medical Subject Heading (MeSH) terms like “neurocysticercosis”, “pathogenesis of NCC “, “comorbidity NCC/AUD” OR “OR “OUD/NCC/DYSBIOSIS”; OR “NCC/AUD/microbiotas” OR “AUD/NCC/Brain damage”.
Results: The total of selected articles were previously peer-reviewed, and we did not find publications related to NCC/AUD, AUD/NCC/Dysbiosis, NCC/AUD/IS/Ep.
Comments and closing thoughts: We delivered some hypotheses on the role played by AUD on NCC and vice versa, as well as on dysbiosis. It is the first time this review has been done.
Keywords
Neurocysticercosis; Alcohol use disorder; Gut microbiome; Gut microbiota; Toll-like receptors; Microbiota-gut-brain axis; Smallchain fatty acids; Neurotransmitters; Hormones; Peptides; Therapeutic targets and interventions; Dysbiosis; Nuclear factor kappa b
List of Abbreviations
(As) Astrocyte; (AUD) Alcohol Use Disorder; (AD) Alzheimer’s Disease; (BBB) Blood-brain-barrier; (CNS) Central Nervous System; (DA) Dopamine; (DR1) Dopamine Receptor D1; (FASD) Fetal Alcohol Syndrome Disorder; (FFAR) Free Fatty Acid Receptor; (GABA) Gamma Amino Butyric Acid; (GF) Germ-free; (GM) Gut Microbiota; (GBA) Gut-brain-axis; (GPCR) G Protein-coupled Receptors; (IL) Interleukin; (LPS) Lipopolysaccharide; (micRNA) Micro Ribonucleic Acid; (Mg) Microglia; (NI) Neuroinflammation; (NFκB) Nuclear Factor-κappa B; (NMDA) N-methyl-D-aspartate; (OS) Oxidative Stress; (PCD) Programmed Cell Death; (PFCX) Prefrontal Cortex; (RCD) Regular Cell Death; (SCFAs) Short Chain Fatty Acids; (SPF) Specific Pathogen-free; (TBI) Traumatic Brain Injury; (TLRs) Toll-light Receptors
Introduction
Cysticercosis (Ct) is a neglected and preventable parasitic zoonosis secondary to an infection in humans and pigs by the larva form of the pork tapeworm Taenia solium (Ts), commonly seen in persons living in developing countries. Cysticercosis can infest most organs in the final host (human) and the intermediate host (pigs). When Ct invades intracranial components or the spinal cord, this process is named Neurocysticercosis (NCC), and its most common clinical manifestations are headache and Epileptic Seizures (ES)/Epilepsy (Ep), mainly in the intraparenchymal invasion [1-20].
We conducted many epidemiological studies in rural areas around Mthatha (South Africa), and we concluded that NCC is the most common cause of secondary epilepsy. On top of that, ES and Ep respond very well to Antiepileptic Drugs (AED) and Antiseizure Medication (ASM) [7- 30]. Likewise, lack of available AEM/AED and poor compliance are the leading causes of Status Epilepticus (SE) in our region. Patients complaining of refractory EP due to NCC were never identified around Mthatha from the past 2 decades [30-40]. The commonly used ASM is benzodiazepine, and the commonest AED are valproic acid and carbamazepine [1-15].
Recently, we published some novel aspects of NCC associated with COVID-19, HIV, autoimmunity, and the role activated OLG/OPC/NG2 plays in the pathogenesis of NCC and its lymphatic drainage. As we documented before, the activation of Microglia (Mg) and Astrocytes (As) are intensely involved in the pathogenesis of NCC, and this year, we commented on the role played by Rouget cells/pericytes in the pathogenesis of NCC [31,32].
Alcohol Use Disorder (AUD) is a current public health problem worldwide, affecting around 10% of its population and responsible for more than 3 million deaths every year [33,34]. USD is a cerebral disorder characterized by an incapacity to control or stop alcohol consumption despite adverse occupational, social, or health consequences. This condition is also named alcohol addiction, alcohol dependence, alcohol abuse or alcoholism and can be severe, moderate or mild.
More than 35,000 bacterial species are present in the human gut microbiota, making anaerobes more prominent. As mentioned, Firmicutes and Bacteroidetes are the most important ones [35]. Some authors have established that external factors like malnutrition (low dietary fibre/vitamins, preservatives, emulsifiers), AUD, some medications, toxins, heavy metals, radiation, and stress favour dysbiosis [36].
The main aims of this study are to evaluate the association between AUD, NCC and dysbiosis from reported cases in the available medical literature, to describe any novel clinical features of patients with AUD/NCC presenting IS/ ES/Ep, and to answer the following research question:
1. How often has this comorbidity been reported in the medical literature?
2. What is the most accepted pathogenesis of AUD/NCC/ dysbiosis?
Material and Methods
Search strategy
A comprehensive literature search using the most critical databases was made to select all articles related to “microbiota,” “alcohol use disorder,” “gut microbiome,” “neurocysticercosis,” “alcohol consumption,” “microbiome,” “dysbiosis,” or “alcohol abuse.”
This study focused on manuscripts published between January 01, 2020, and August 31, 2024, to ensure the identification of recent studies while capturing relevant developments in the field. Researchers who investigated the impact of alcohol on gut dysbiosis or modifications in the composition and functions of the gut microbiotas in patients with AUD were also included. Both authors thoroughly studied the identified publications, and conclusions, methodologies, and key findings were extracted coherently using a Microsoft Excel program and organizer.
We used a novel search strategy for the selected databases, registers, and websites, including filters and limits. We perform this study using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Database retrieval and data extraction were performed independently by 2 authors. Subject headings and text words were searched in electronic databases such as Scopus, Central PubMed, Web of Science, Medline (Ovid), and EMBASE. We also searched the medical publications looking for published Medical Subject Heading (MeSH) terms like”, “pathogenesis of AUD/NCC”, “comorbidity AUD/NCC”, or “Management of AUD” OR” AUD/NCC/ Ep/ES” OR “NCC/AUD/Cerebrovascular diseases”.
We also checked https://www.clinicaltrials.gov/, from the US National Library of Medicine, looking for unpublished studies, using the same MeSH terms above mentioned, but applying the filters “entire articles”, “full publication”, and “abstract”, published in Spanish, English, or Portuguese.
The reported incidence and prognosis of NCC/AUD in the medical literature were searched. Weighted Mean Differences (WMDs) with referent 95% Confidence Intervals (CIs) and pooled Odds Ratios (ORs) with 95% CIs were assessed to explore the risk factors of AUD/NCC/ IS/Ep. The references of selected manuscripts and relevant reviews were also screened, and additional reports were looked for.
Eligibility criteria
Separately, all authors reviewed each publication, screening the title and abstract and, in most cases, reading the whole manuscript. Publications were included if they raised concerns. We only selected articles published in Spanish, English, or Portuguese. When overlapping cohorts were present, we chose the manuscripts with the best calculations and larger sample sizes. Other publications were excluded, such as letters to editors, editorials, and conference proceedings.
Data collection and risk of bias assessment
We planned to extract the study location, confirmation of diagnosis, study design, patient management, potential risk factors, year of publication, sample size, prognosis, and demographic features from the chosen publications. To assess the quality of the manuscript, we used the Newcastle Ottawa Scale (NOS) scoring for high-quality research, like ≥ 7, as the selective criteria. All disagreements were resolved through bilateral scientific discussion until we reached a unanimous consensus.
Statistical analysis
Our protocol establishes that the statistics analysis for this study is performed using Review Manager version 5.4 from Cochrane Collaboration, Oxford, United Kingdon. Notwithstanding, we converted to mean and Standard Deviation (SDSD) all continuous variables presented by median (quartile) or median (range) according to the reported formula. In some reports, using the Mantel– Haenszel fixed-effects, we Weighted Mean Differences (WMDs) with corresponding 95% CIs and pooled ORs with 95% CIs were calculated when risk factors were identified in more than one investigation. In the absence of heterogeneity, the random effect was selected.
Exclusion and inclusion criteria.
All articles suitable for this study meet the following inclusion criteria:
1. Previous ethical approval for all released information was requested.
2. Articles written in English, Spanish, or Portuguese.
3. The central aspects must be NCC, AUD, and the pathogenesis of AUD/NCC.
4. The manuscript must be published in a peer-reviewed medical journal.
The mandatory exclusion criteria were:
1. Publication did not refer to issues numbered three.
2. Letters to the editor, medical hypotheses, review articles, medical newspaper and all articles that did not match the criteria of an original study.
3. Conference proceedings;
4. Clinical trials with less than 15 patients per treatment arm;
5. Duplicate articles and manuscripts written by the same author with the same data process;
6. Publication excluding the corresponding authors. All papers were screened at least twice (blinded). All manuscripts presenting exclusion criteria were not selected.
Medical literature searching programmed
We selected observational cohort studies, systematic reviews and meta-analyses, case reports, case series, Randomized Controlled Trials (RCTs), cross-sectional studies, and published guidelines that were first identified to provide the most relevant Level Of Evidence (LOE), if available. Appropriate investigations were displayed in a standardized format, with the quality of each study graded using the Oxford LOE (LOE Level 1a–5). During this search, we looked for articles published between January 01, 2015, and December 31, 2023. We searched the databases: Web of Science, Medline (Ovid), Scopus, Central PubMed, and EMBASE. We also included articles from other databases such as Google Scholar, online databases, Scielo, BioRxiv, medRxiv and Cochrane Library. All studies were retrieved by utilizing MeSH, as previously cited.
Study selection
We selected case reports, case series, cohort studies, clinical trials, case-control studies, review articles, meta-analysis reporting data on listed topics and controlled clinical trials. Two studies that meet the inclusion criteria were excluded because the authors did not provide proof of confirmation of associated comorbidity. A flow diagram shows the number of searched articles, the number of selected manuscripts, the selected exclusion criteria, and the total identified study.
Process of data collection
Both authors independently used Microsoft Excel to extract the most relevant information from each article. The data obtained included
• clinical features,
• population size,
• age distribution,
• the investigations used to diagnose NCC,
• clinical assessment for AUD, and
• other investigations considered to confirm the diagnosis of AUD and its therapy
Synthesis of data
Our study used aggregate data where possible, following the PRISMA recommendations.
Quality assessment of included manuscripts
We employed the modified Jadad scoring system to assess blinding outcomes and the bias [37]. We separately assessed each publication with the same automation tools for risk of bias using the Jadad scoring system (based on randomization-2, double-blinding-2, and a description of dropout rate-1) [15]. Trials scoring 3 or greater were considered good-quality trials (Jadad’s scores range/from 0 to 5). Therefore, trials with a Jadad score <3 were not included.
Results
Study selection
2095 manuscripts were retrieved from electronic databases until August 31, 2024. After removing irrelevancy and duplicates, 308 papers were taken for full-text screening, and finally, 6 studies reporting outcomes of interest were included for review. All selected manuscripts were peerreviewed, but no publications were found, including NCC/ AUD/Ep/IS. A flow chart from the literature search is shown in Figure 1.
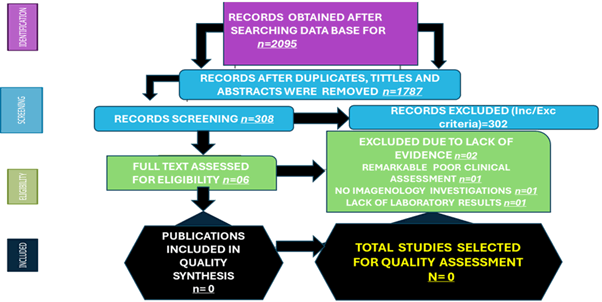
Figure 1: Flow diagram with included publications
No patients presenting NCC/AUD were identified. The frequency of AUD in NCC patients was not reported. The mortality rate was not published. No studies reported the incidence/prevalence of NCC/AUD/Ep/IS.
Study and subject characteristics
The USA conducted the most studies (n=2), followed by Korea, China, India, and Italy (n=1 each). The methodological quality of the 6 studies was assessed using NOS, and all studies were generally considered of mediumto- high quality. Still, no report about the comorbidity of OSAS/NCC was reported.
Patient-specific variables
Six studies investigated IS risk factors in patients with AUD. Combining the results of 2 studies, we observed that age at AUD diagnosis was not associated with NCC. No correlation was observed between age at NCC and AUD.
AUD/NCC-specific variables
No significant differences were observed in the 6 studies investigating risk factors for IS/AUD. We did not perform a meta-analysis because the criteria for positivity varied across studies and because no one has the inclusion criteria for comorbidity of AUD/NCC.
Discussion
To answer the first research question, we confirmed that the comorbidity of AUD/NCC/dysbiosis had yet to be reported in the medical literature before this research. Some concepts are highlighted in Figures 2-4, represented below.

Figure 2: Definitions-1

Figure 3: Definitions-3
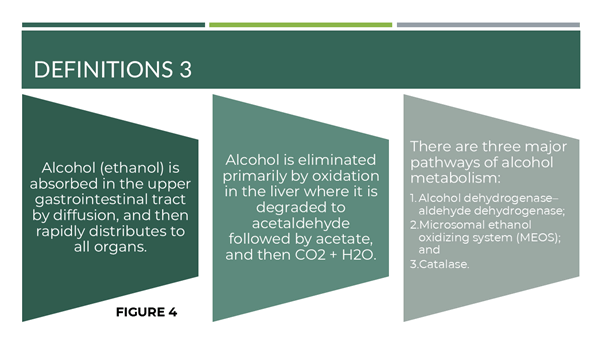
Figure 4: Definitions-3
Brief comments on dysbiosis, NCC and AUD
Maintaining physiological homeostasis is usually seen as a bidirectional communication between the brain and the gut microbiota, better known as the Gut-brain Axis (GBA). Brain maturation and gut microbiota development must be synchronized and follow the same timeline through all age groups. On the other hand, the misbalance between the early development of mesolimbic reward circuitry (dopamine) and the delayed inhibitory prefrontal cortical neurons leads to risk-taking behaviours and increased acquirement of reward-seeking in younger people with an increased risk of substance abuse which can explain the higher incidence of AUD in this age group, despite the underlying mechanism is not clear cut understanding seems to be that it plays a crucial role in AUD neurodevelopment years later. Furthermore, AUD induces dysbiosis, neuro-inflammatory response and neurodevelopmental impairment due to the impact caused by alcohol over the myelination process, mainly during the synaptic pruning of the prefrontal cortex, the limbic cortex and the striatum responsible for motivational behaviours, including cognitive functions [38].
AUD is a chronic brain disorder of unknown aetiology associated with microglia expression and NI, including an impaired capacity to stop or moderate drinking under any circumstances. One hundred trillion commensal microbiotas (also named microbiome) constitute the ecosystem which colonized the human intestine since the neonatal period throughout life and encodes one hundred more genes than the human genome and regulates the adaptive and innate immune system, fermentation of otherwise indigestible carbohydrates and fibres, energy production, synthesis of several vitamins (e.g., vitamins K and B), the metabolism of bile acids, sterols, food digestion, and xenobiotics. The Gut Microbiome (GM) also produces and releases choline, including its metabolites, neurotransmitters, and Shortchain Fatty Acids (SCFAs), into the lumen of the intestine and carried to other organs, including the brain, transported across the epithelial barriers via blood flow. The GM is also known as a new metabolic organ due to its influences on human metabolism, mental health, brain homeostasis, neuronal maturation, brain development, and the immune system [38].
We hypothesized before on the role of GM over the brain via afferent pathways of the vagus nerve (GBA) in patients presenting NCC/COVID-19 leading to brainstem dysfunction under homeostatic conditions including Microglia (Mg) expression and permeability of the BBB supported by Toll-like receptors as facilitator of the communication between the brain and the GM [39-41]. We also hypothesized on the role played by SCFAs regulating the peripheral gut functions, its permeability, and immune expression via Mg polarization into M1 and M2, which can make a remarkable change of the neuronal network, leading to dysfunctional patterns of the CNS aggravated in cases presenting AUD.
In previous publications, we speculated about the role of Mg in patients with NCC as innate immune cells of the CNS in response to the colloid stage of this zoonotic parasitic disease and dysbiosis. Now, we hypothesized that GBA regulates Mg maturation/polarization, and this process can aggravate immunological patients’ response deficiency when AUD is also present, considering that alcohol disrupts Mg maturation and development after dysbiosis.
We hypothesized that in patients presenting NCC/AUD/ Dysbiosis (Dys), Mg abnormalities can be present, which might exhibit less expression when challenged with LPS, increased density and distribution across several brain areas around the colloid cystic lesion favouring MG polarization affecting the neuronal network, functional connectivity and behaviour and leading to alteration of the synaptic plasticity and neurogenesis from neuronal PCD and MG phagocytosis even beyond the Mg phenotype is restored. We also speculated on the role of AUD on the Mg expression through dysbiosis caused by uncontrolled alcohol intake, leading to dysfunctional neuroimmune system and neurodegeneration.
The harmful alcohol intake causes around 5.3% mortality rate in all age groups, including an alarming rate of 13.5% in the age group of 20 years-39 years old, while the relationship between AUD and GM has been well documented recently. On the other hand, an increased content of Gram-negative anaerobic bacteria has been found in the gut of alcoholic persons compared with a control group, leading to dysbiosis, which has been well documented in cases of AUD, PD, AD, autism spectrum disorder and depression [Getachew]. We hypothesized that in cases of NCC/AUD/Dysbiosis, some metabolite of GM, such as SCFAs, cross the BBB and injure the Mg, leading to Mg polarization, causing NI and breakdown of the BBB, OS, disrupting Mg maturation, leaky gut (microorganism passing into the lumina) and its consequences with the participation of toll-like receptors.
Prolonged and heavy exposure to alcohol can cause a remarkable decrease in the number of glial cells in both frontal and temporal cortices and neuron functionality with more intensity at the cerebellum, corticolimbic system and cerebellar vermis (white matter) [42,43].
However, chronic high alcohol exposure increases Mg expression, which is associated with increased levels of pro-inflammatory cytokines, chemokines, and OS (ROS), leading to PCD/RCD and tissue damage [44,45]. Chemical depletion of Mg blocks the production of pro-inflammatory cytokines in the brain after acute binge ethanol withdrawal [46].
Based on some studies on fetal alcohol syndrome disorders, the role played by Mg in the neurodevelopment mechanism has been confirmed mainly in regions where its process depends directly on the neuroglial cell’s migration, such as the anterior commissure and corpus callosum. Nevertheless, AUD can lead to micro-encephaly during the brain spurt, typically characterized by a fast growth, maturation, and proliferation of the glial cells [38]. We hypothesized that Mg can be a crucial mediator of alcohol behavioural effects based on the previous statements and considering that Mg can interfere with homeostasis by diminishing the activity of homeostatic genes due to alcohol activity on Toll-like receptors as a crucial mediator of inflammatory pathways in the gut. As we hypothesized before, NCC TLR (belongs to pattern recognition receptors-PRRs) play a remarkable role in keeping the balance between the mucosa immune system and the intestinal commensal bacteria for its first line of defence against pathogens and can identify the conserve pathogen-associated molecular patterns (conserve structures of pathogens) [46]. As we reported before, PRR signalling can trigger the release of proinflammatory cytokines, PCD/ RCD, Mg expression and adaptive immune response as part of the active mechanism to eliminate malignant cells and infectious pathogens [25,27,31]. We hypothesized that in AUD/NCC/dysbiosis comorbidity, both types of 28 TLRs (cell membrane/intracellular) mainly (TLR4) are expressed in Mg/As, and they can trigger the induction of genes able to encode cytokines via downstream stimulation of nuclear factor-kB as is shown in Figure 5.
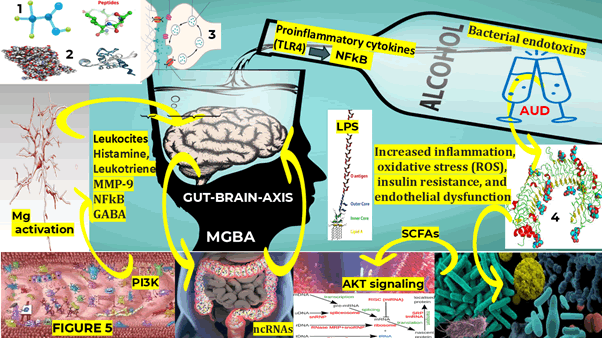
Figure 5: Representation of the interrelationship between the microbiota gut brain axis, neuroinflammation, oxidative stress with the participation of the nuclear factor kappa B, lipid polysaccharide and microglia polarization and the association of non-coding RNAs and m6A of ncRNAs with AUD/NCC/Dysbiosis. At the first phase the m6A modification is modulated by demethylases (eraser) and methyltransferase (writers) triggered by NI and OS. We hypothesized that in patients presenting AUD/NCC/Dysbiosis the ncRNAs and M6A-ncRNAs joined to some molecules leads to the poor prognosis of some patients due to dysfunctional RNA/mRNA. In this figure are excluded other important m6A functions including heterochromatin formation by histone methyltransferase to provide better understanding of the representation
Based on the investigations done by other authors, we hypothesized that NI is remarkably present in AUD/NCC/ Dysbiosis, and it can be identified by confirmation of high levels of C-reactive protein [47].
As has been well established before by other authors, the role played by the microbiota-gut-brain axis in maintaining the anatomical structure and network function of the CNS through the small-molecule Short-chain Fatty Acids (SCFAs), neurotransmitters, peptides and hormones cannot be underestimated [48]. These mechanisms should be considered for the best control of AUD-related gut dysbiosis, astrogliosis and cleaning brain function, gut leakiness, neurodegeneration and NI, as reported by the same authors and we represented in Figure 5 [48].
On the other hand, it’s well known that the primary cause of liver damage is caused by chronic alcohol consumption and its direct effect on the gut microbiome, and remarkable changes in a variety of bacteria leading to bacterial overgrowth has been commented by some authors recently including the beneficial impact of Fecal Microbiota Transplantation (FMT) in patients presenting Alcohol Liver Disease such as steatohepatitis, steatosis, liver cirrhosis and hepatocellular carcinoma [49]. At this level, we would like to highlight one more time the harmful effect alcohol over the brain function via MGBA through the afferent fibres of the vagal nerves carrying bacterial metabolites which cross the BBB and activate the NFκB in the glial cells, causing damage to the neuronal network and accumulation of the TNF-alpha leading to MG polarization and its consequences reported before. We hypothesized that in cases of AUD/NCC/Dysbiosis, many bacterial species can produce dopamine, GABA, and ammonia and decrease levels of SCFA (butyrate) that also cross the BBB, leading to histone modifications, neuropsychiatric manifestations, As PCD and cognitive decline associate with NCC/ dysbiosis. Some of the before-cited disorders may respond positively to anti-inflammatory cytokine (IL-10), dietary interventions (prebiotics/probiotics), AUD medications such as acamprosate, disulfiram, naltrexone and FMT [39,50].
As we previously documented, the CNS major inhibitory neurotransmitter (GABA) synthesized by GABAergic neurons and supporting cells from glutamate is released into the synaptic cleft, activating surrounding GABA receptors under neurophysiological and neuropathological conditions. Among those cells, Astrocytes (As) are the primary homeostatic glial cells in the CNS and regulate synaptic neurotransmission and GABA homeostasis, and we hypothesized that As dysregulation is deeply implicated in NCC/AUD/Dys as has been reported by other authors in conditions such as major depressive disorders [50].
Recently, we documented the role of Mg in patients presenting NCC. Now, we are to comment on some aspects of the interrelationship between Mg and AUD. These cycles of alcohol abuse, withdrawal and relapse, known as AUD, have been proven to be associated with neuroadaptations in the lentiform nucleus and ventral pallidum related to reward processing, drug-seeking behaviours, and habit formation mainly when the Mg cells at the basal ganglia are dystrophic secondary to AUD. At the same time, the As remains healthy [51].
On the other hand, Schuch, et al. (2023) have highlighted that AUD, through epigenetic changes, can change gene activity mainly through Global DNA methylation (levels of 5-methylcytosine), provoking remarkable influences on hormonal and environmental effects. Based on the previous statement, we hypothesized that the impact caused by heavy and chronic alcohol intake on Global DNA methylation is one of the leading causes of brain damage related to AUD [52].
Noncoding RNA (ncRNA) is a functional RNA molecule not translated into a protein. According to Nakashima and collaborators, many studies have documented the activity of ncRNAs in the CNS of patients presenting AUD, supporting the hypotheses of the closed relationship between genetic factors and alcohol dependence. Therefore, dysregulation of the ncRNAs is always present in AUD, aggravated by gut dysbiosis, as we graphically represented in Figure 5 [53,54].
It has to highlight the role played by SCFAs (butyrate/ propionate) as mediators of the interaction between eating/ drinking and the stress response through the MGBA and regulation of the histone acetylation process, and we suspect the same happens in patients with NCC and associated SARS-CoV2 infection as happened during the COVID-19 pandemic [28,29,53]. An associated AUD might affect the bidirectional homeostatic interrelationship via the epigenetic mechanism of several metabolites, such as propionate and butyrate. We also speculate that in AUD/ NCC/Dysbiosis cases, several miRNAs with epigenetic mechanisms play a crucial role in the most remarkable neurophysiological homeostatic mechanisms.
Conclusion
Therefore, regulating the host miRNA by SCFAs (microbial butyrate/acetate), the effect (NI) exerted by secreted glutamate at the pericystic colloid stage of the NCC will be increased or decrease accordingly if the interaction of gut microbiota and regulatory ncRNA (circRNA, miRNA, lncRNA). Remember that gut dysbiosis is caused by too much alcohol consumption by reducing several peptides and mucus, damaging the intestinal barrier, changing the microbiota composition, and activating inflammatory mediators-NFκB signalling pathway leading to PCD/RCD.
Declarations
Consent for publication
The authors did not request informed consent because it was unnecessary for this study review.
Availability of data and material
The data supporting this study are publicly available on reasonable request from the corresponding author.
Ethical Approval
This manuscript did not require ethical approval.
Acknowledgement
Thanks to Prof Thozama Dubula and Dr Sibi Joseph for their continued encouragement and unconditional support. We thank the authors whose articles have been cited as references in this manuscript.
Competing Interest
The authors certified that they made this review without any issue able to construe a potential conflict of interest.
Funding
The authors report that they did not receive any external personal collaboration or financial support to influence the results written in this manuscript.
Study concept and design: LFIV and HFS.
Data collection: LdeF IV and HFS. Data analysis: LdeFIV and HFS. Drafting of the manuscript: HFS and LFIV. Revising the manuscript: HFS. Supervising research and manuscript writing process: LDFIV and HFS. Both authors have approved the publication of this article.
Contributors
Acquisition of data or analysis and interpretation of data: LIV. Drafting this paper/critically revising the manuscript for important intellectual content: LIV, HFS. All authors are the guarantors, and because they have contributed equally to this work, they share their first authorship.
References
- H.F. Sibat, L.F.I. Valdes, Pseudo seizures and epilepsy in neurocysticercosis, Electron J Biomed, 2(2003):79-87.
- H.F. Sibat, M.D.I.V. LdeF, Vascular dementia type binswanger's disease in patients with active neurocysticercosis, Rev Electron Biomed,1(2003):32-42.
- F.S.H.I.V. LdeF, Insular neurocysticercosis: Our finding and review of the medical literature, Inte J Neurol, 5(2006):2.
- H.F. Sibat, L.D. Cowan, H. Carabin, I. Targonska, M.A. Anwary, et al. Accuracy of serological testing for the diagnosis of prevalent neurocysticercosis in outpatients with epilepsy, Eastern Cape Province, South Africa, PLoS Negl Trop Dis, 3(2009):e562.
- H.F. Sibat, I.V. LdeF, J. More-Rodriguez, Parasitic zoonoses of the brain: Another challenger, Inte J of Neurol, 12(2009):9-14.
- H.F. Sibat, I.V. LdeF, Treatment of epilepsy secondary to neurocysticercosis, Novel Treat Epilepsy, InTech, 2011.
- H.F. Sibat, L.F.I. ValdÃÂ?©s, Clinical features of epilepsy secondary to neurocysticercosis at the insular lobe, Novel Asp Epilepsy, InTech, 2011.
- H.F. Sibat, Epilepsy secondary to parasitic zoonoses of the brain, Novel Asp Epilepsy, InTech,2011.
- H.F. Sibat, M. Salazar-Campos, L. Ibanez-Valdes, Cysticercosis of the extraocular muscles: Our experience and review of the medical literature, Internet J Neurology, 14(2001):1.
- H.F. Sibat, L.F.I. Valdes, Introduction to cysticercosis and its historical background, InTech, (2013).
- H.F. Sibat, L.F.I. Valdes, What is a low frequency of the disseminated cysticercosis suggests that neurocysticercosis is going to disappear? InTech, (2013).
- H.F. Sibat, L.F. Valdes, Uncommon clinical manifestations of cysticercosis, InTech, (2013).
- L.F.I. Valdes, H.F. Sibat, Psychogenic nonepileptic seizures in patients living with neurocysticercosis, InTech open, (2018).
- L.F.I. Valdes, H.F. Sibat, Subarachnoid cysticercosis and ischemic stroke in epileptic patients, InTech open, (2018).
- E.V. Noormahomed, N. Nhancupe, J. Mufume, R.T. Schooley, H.F. Sibat, et al. Neurocysticercosis in epileptic children: An overlooked condition in Mozambique, challenges in diagnosis, EC Microbiology, 17(2021):49-56.
- H.F. Sibat, Racemose neurocysticercosis long COVID and brainstem dysfunction: A case report and systematic review, Clin Schizophr Relat Psychoses, 15S(2021).
- H.F. Sibat, Neurocysticercosis, epilepsy, COVID-19 and a novel hypothesis: Cases series and systematic review, Clin Schizophr Relat Psychoses, 15(2021).
- H.F. Sibat, People living with HIV and neurocysticercosis presenting Covid-19: A systematic review and crosstalk proposals, Clin Schizophr Relat Psychoses, 15(2021).
- H.F. Sibat, L.F.I. Valdes, Novel hypotheses on the role of oligodendrocytes in neurocysticercosis: Comprehensive review, Clin Schizophr Relat Psychoses, 17(2023).
- H.F. Sibat, Comorbidity of neurocysticercosis, HIV, cerebellar atrophy and SARS-CoV-2: Case report and systematic review, Clin Schizophr Relat Psychoses, 15(2022).
- A.H. Del Rio-Romero, H.F. Sibat, Prevalence of epilepsy and general knowledge about neurocysticiercosis at Nkalukeni Village, South Africa, Int J Neurology, 3(2004).
- H.F. Sibat, A.H. Del Rio-Romero, Neuroepidemiological survey for epilepsy and knowledge about neurocysticiercosis at Ngqwala Location, South Africa, Int J Neurol, 3(2005):11-16.
- H.F. Sibat, A. Del Rio-Romero, L. Ibanez-Valdes, Prevalence of epilepsy and general knowledge about neurocysticercosis at Ngangelizwe Location, South Africa, Int J Neurol, 4(2005):23-37.
- A. Del Rio, H.F. Sibat, Epidemiological survey about socio-economic characteristic of Mpindweni Location, South Africa, Int J Neurol, 4(2005):18-26.
- H.F. Sibat, L.F.I. Valdes, Refractory epilepsy in neurocysticercosis, Int J Neurol, 5(2006):1-6.
- L.F.I. Valdes, H.F. Sibat, Meningeal lymphatic vessels and glymphatic system in neurocysticercosis: A systematic review and novel hypotheses, Clin Schizophr Relat Psychoses, 17(2023).
- L.F.I. Valdes, H.F. Sibat, Co-morbidity of spinal cord neurocysticercosis and tuberculosis in a HIV-positive patient, Int J Neurol, 7(2007):5-10.
- H.F. Sibat, L.F.I. Valdes, The role of pericytes in neurocysticercosis: Comprehensive review and novel hypotheses, Clin Schizophr Relat Psychoses, (2013).
- H.F. Sibat, L.F. Valdes, Novel hypotheses on the role of microglia and panoptosis in neurocysticercosis, Clin Schizophr Relat Psychoses, 17(2023).
[Crossref]
- F.S. Humberto, Bilateral putaminal haemorrhage and blindness in times of the coronavirus pandemic and dysbiosis: Case report and literature review, Clin Schizophr Relat Psychoses, 15S(2021).
- H.F. Sibat, L.F. Valdes, New hypotheses on the role of microglia in ischemic reperfusion injury secondary to neurocysticercosis and a comprehensive review, Clin Schizophr Relat Psychoses, (2023).
- M.N. Garcia, H.F. Sibat, L.F.I. Valdes, Our hypotheses about the role of cuproferropanoptosis in neurocysticercosis and a comprehensive review, J Drug Alc Res, 12(2023).
[Crossref]
- H.F. Sibat, L.F.I. Valdes, D. Thozama, Neuropsychiatric manifestation in neurocysticercosis, J Drug Alc Res, (2023).
- H.F. Sibat, L.F.I. Valdes, Ferropanoptosis in neurocysticercosis: A comprehensive research, J Drug Alc Res, 13(2023):1-10.
- L.F.I. Valdes, The Role of rouget cells in neurocysticercosis, J Drug Alc Res, 13(2024).
- T. Hoang-Anh, Q. Duong-Minh, N. Nguyen-Thi-Y, S. Duong-Quy, Study of the obstructive sleep apnea syndrome in cerebral infarction patients, Front Neurol, 14(2023):1132014.
- H.F. Sibat, L.F.I. Valdes, The oxidative stress in neurocysticercosis, J Drug Alc Res, 13(2024):1-15.
- World Health Organization, Global status report on alcohol and health, (2018).
- R. de Beaurepaire, P. Jaury, Baclofen in the treatment of alcohol use disorder: Tailored doses matter, Alcohol Alcohol, 59(2024):agad090.
- I. Koutromanos, E. Legaki, M. Gazouli, E. Vasilopoulos, A. Kouzoupis, et al. Gut microbiome in alcohol use disorder: Implications for health outcomes and therapeutic strategies-a literature review, World J Methodol, 14(2024):88519.
- A.R. Jadad, R.A. Moore, D. Carroll, C. Jenkinson, Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials, 17(1996):1-12.
- J.F. Masa, J.L. Pepin, J.C. Borel, B. Mokhlesi, P.B. Murphy, et al. Obesity hypoventilation syndrome, Eur Respir Rev, 28(2019):180097.
- B. Getachew, S.R. Hauser, S. Bennani, N. El Kouhen, Y. Sari, et al. Adolescent alcohol drinking interaction with the gut microbiome: Implications for adult alcohol use disorder, Adv Drug Alcohol Res, 4(2024):11881.
- J.Y. Yang, X. Xue, H. Tian, X.X. Wang, Y.X. Dong, et al. Role of microglia in ethanol-induced neurodegenerative disease: Pathological and behavioral dysfunction at different developmental stages, Pharmacol Ther, 144(2014):321-37.
- S.M. De La Monte, J.J. Kril, Human alcohol-related neuropathology, Acta Neuropathol, 127(2014):71-90.
- J. Zhao, Z. Lv, F. Wang, J. Wei, Q. Zhang, et al. Ym1, an eosinophilic chemotactic factor, participates in the brain inflammation induced by Angiostrongylus cantonensis in mice, Parasitol Res, 112(2013):2689-95.
- L. Qin, F.T. Crews, NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration, J Neuroinflamm, 9(2012):5-19.
- E.N. Grodin, L.R. Meredith, E.M. Burnette, K. Miotto, M.R. Irwin, et al. Baseline C-reactive protein levels are predictive of treatment response to a neuroimmune modulator in individuals with an alcohol use disorder: A preliminary study, Am J Drug Alcohol Abuse, 49(2023):333-344.
- L. Xie, W. Rungratanawanich, Q. Yang, G. Tong, E. Fu, et al. Therapeutic strategies of small molecules in the microbiota-gut-brain axis for alcohol use disorder, Drug Discov Today, 28(2023):103552.
- J.T. Wolstenholme, N.K. Duong, E.R. Brocato, J.S. Bajaj, Gut-liver-brain axis and alcohol use disorder: Treatment potential of fecal microbiota transplantation, Alcohol Res, 44(2024):01.
- D.N. Ali, H.M. Ali, M.R. Lopez, S. Kang, D.S. Choi, Astrocytic GABAergic regulation in alcohol use and major depressive disorders, Cells, 13(2024):318.
- A.E. Rasool, T. Furlong, A.A. Prasad, Microglia activity in the human basal ganglia is altered in alcohol use disorder and reversed with remission from alcohol, Addict Biol, 29(2024):e13374.
- J.B. Schuch, C.E. Bandeira, J.L.S Junior, D. Muller, M.F. Charao, et al. Global DNA methylation patterns in SS, Genet Mol Biol, 46(2023):e20230139.
- M. Nakashima, N. Suga, S. Yoshikawa, Y. Ikeda, S. Matsuda, Potential molecular mechanisms of alcohol use disorder with non-coding rnas and gut microbiota for the development of superior therapeutic application, Genes (Basel), 15(2024):431.
Copyright: © 2024 Lourdes de Fatima Ibanez Valdes, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

