Research Article: Journal of Drug and Alcohol Research (2022) Volume 11, Issue 8
Cytotoxicity and Immunomodulatory Effects of Propolis on Cancer and Normal Cells
Maha H. AbdullKadem1, Entisar J. Al-Mukhtar1 and Kaiser N. Madlum2*2Department of Human Anatomy, University of Babylon, Iraq
Kaiser N. Madlum, Department of Human Anatomy, University of Babylon, Iraq, Email: Kaiser.madlum@uobabylon.edu.iq
Received: 01-Aug-2022, Manuscript No. jdar-22-73248; Editor assigned: 03-Aug-2022, Pre QC No. jdar-22-73248 (PQ); Reviewed: 17-Aug-2022, QC No. jdar-22-73248; Revised: 22-Aug-2022, Manuscript No. jdar-22-73248 (R); Published: 29-Aug-2022, DOI: 10.4303/jdar/236191
Abstract
Leukemia is a neoplastic proliferation of one kind of blood cells, characteristically a leukocyte. Leukemia is a set of malignant illnesses affecting the blood and blood-forming tissues including bone marrow, lymphatic system, and spleen. Propolis is a natural resinous product that honeybees collect from several plants and mix with beeswax and salivary enzymes. It contains a complex mixture of organic and non-organic compounds. Propolis has numerous uses in folklore medicine for healing many types of diseases. The objective of the current study was to determine the cytotoxic and immunomodulatory effect of aqueous and ethanolic extracts of propolis on cancer cells (lymphoma cell line) and normal cells (peripheral blood lymphocytes PBLs). The immunomodulatory effect was assessed by measuring the level of IL-4, IL-10, IL-17, and IFN-ℽ.
Material and method: cancer (Raji cell line) and normal cells (PBLs) were treated with different concentrations (1000 to 31 µg/ml) of both aqueous and ethanolic extracts of propolis to determine the effect of propolis extracts on the viability of these cells by using MTT assay. ELISA assay kits were used to measure the level of IFN-ℽ, IL-4, IL-10, and IL-17 in the supernatant of the cells.
Results: Higher concentration of ethanolic and aqueous extracts of propolis caused a decrement in the viability of the Raji cell line but did not affect the viability of the PBLs cells. Regarding the immunomodulatory effect of propolis' extracts, on the Raji cell line, aqueous extract at concentrations (50, 250 and 1000 µg/ml) and ethanolic extract at the concentration (1000 µg/ml) caused a decrease in the level of IL-4. Ethanolic extract at concentration 1000 µg/ml caused a decrease in the level of IL-10, while at concentration 50 µg/ml of the same extract caused an increase in the level of IL-10. All concentrations of both aqueous and ethanolic extracts of propolis caused a decrease in the level of IL-17 and IFN-ℽ. Regarding the effect of propolis extracts on PBLs, both aqueous and ethanolic extracts of propolis in concentrations (250 and 50 µg/ml) caused an increment in the level of IL-4, and IL-17, while ethanolic extract at concentration 1000 µg/ml caused a decrease in the level of these cytokines. Propolis aqueous and ethanolic extracts at concentration 250 µg/ml caused a significant elevation in the level of IL-10. Aqueous extract at concentrations 50, 250 µg/ml and ethanolic extract at concentration 1000 µg/ml caused a significant increase in the level of IFN-ℽ.
Conclusion: Propolis promotes PBLs proliferation and had an antiproliferative effect against the Raji cell line. It had a dose-dependent immunomodulatory effect.
Keywords
Propolis; Raji cell line; Peripheral blood lymphocytes; Cytokines
Introduction
Propolis is a natural product that is collected by honey bees from various plants. There are several types of propolis depending on the plant and geographical zone from which it is collected [1]. Cancer is the uncontrolled growth of abnormal cells anywhere in the body. These cells can infiltrate normal body tissues [2]. Cancer metastasis is a process in which malignant cells disperse from the primary tumor, where they settle and grow at a site other differ from the primary cancer site, about 90% of cancer deaths are due to cancer metastasis rather than primary cancer [3]. Leukemia is a group of malignant diseases affecting the blood and blood forming tissues including bone marrow, lymphatic system, and spleen [4]. Burkitt’s lymphoma is a fast rising non-Hodgkin’s lymphoma. The causes of this type of lymphoma include virus infection or chromosome rearrangement, but the accurate causes remain unknown. Treatment for this disease is chemo and radiotherapy. It remains to be the highly aggressive and incurable B-cell lymphoma despite the current therapy [5]. One of the crucial functions of leukocytes in the production of cytokines, a small secreted by different types of cells and has different effects on the interactions between cells. Cytokines have critical role in the growth and differentiation of normal and leukemic cells, they have a major role in leukemogenesis. Prevention of apoptosis by the abnormal production of a cytokine may release the cell from normal growth control leading to malignant transformation.
Materials and Methods
Cell line
Raji cell line was kindly provided from the Tissue Culture Laboratory in the College of the Medicine/University of Babylon. Cells were cultured in a (RPMI-1640) medium supplemented with 10% fetal bovine serum.
Peripheral blood mononuclear cells separation
The separation of mononuclear cells was done through density gradient centrifugation. Briefly, five ml of venous blood was withdrawn from healthy 25-30 years donors, left for 30 minutes to be cooled to room temperature. The blood was carefully and slowly added to 5 ml of density gradient medium (Lymphoprep,1.077 gm/ml), without mixing, then the tubes were centrifuged at 400 g for 30 minutes. PBMNs cloudy band were transferred into another tube, washed twice with phosphate buffer saline, and centrifuged for 400 g for another 10 minutes. Pellets then suspended in RPMI supplemented with 10% FBS in T25 flask and incubate overnight at 37°C and 5% CO2 [6].
Preparation of propolis extracts
The Propolis sample was obtained from a local apiary in AL-Hilla city and identified by Al-Qassim green university/ college of agriculture. Ten grams of propolis were soaked either in 200 ml of 99% ethanol or in 200 ml of double distilled water and left for 48 hours at room temperature. The solutions were filtered using Whitman filter paper no.1, and then evaporated using a rotary evaporator to obtain clear dry powder extract. The stock solutions of propolis were made by dissolving 8.5 mg of aqueous extract powder in 4.25 ml of serum-free RPMI 1640, then the solution was sterilized by filtration using a Millipore syringe filter (0.22 um).
Cytotoxicity assay
Raji cells and human PBLs were seeded in 48 tissue culture plates. The Control group received 500 ml/well of cell suspension in RPMI complete medium while treatment groups received 500 ml/well of the cells suspended in RPMI complete medium containing serial dilutions (1000, 500, 250, 125, 62.5, and 31.25 μg/ml) of each aqueous and ethanolic extracts of propolis. Then the plates were covered with a self-plate and incubated for 24 hours, after that, the cells viability protein was assessed by MTT cytotoxicity assay.
Cytokines production assessment
Each of the Raji cells and PBLs were seeded in 48 tissue culture plates. After the treatment with different concentrations of the two types of extract, the cells were incubated for 24 hours, and then the cell suspension in each well was transferred to the Eppendorf tube and centrifuge at 400 g. The supernatant of each well was transferred to another tube and frozen at -20°C until used. Measurement of the concentrations of IL4, IL10, IL17, and IFN-gamma was done by the ELISA technique.
Statistical analysis
Data were collected, arranged and analyzed using Microsoft Office Excel 2019 and Sigma plot v 12.5. One-way ANOVA test was used to estimate significant differences among the data means. P-value (p ≤ 0.001 and p ≤ 0.05) were considered statistically highly significant and significant respectively.
Results
Cytotoxicity of propolis extracts on Raji cell line
Aqueous extract of propolis caused a highly significant decrease in Raji cell viability at concentrations above 250 μg/ ml. While the ethanolic extract decreases the viability of the Raji cell only at a concentration of 1000 μg/ml (Figure 1).
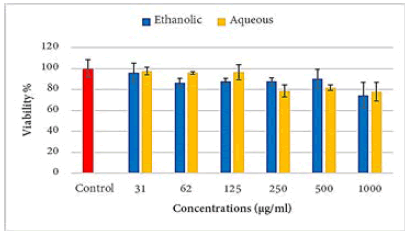
Figure 1: Effects of propolis extracts of on the viability of the Raji cell line
Cytotoxicity of propolis extracts on isolated peripheral blood lymphocytes
Aqueous extract showed a statistically highly significant (p ≤ 0.001) increment in the viability of PBLs at the concentrations of 1000 and 500 μg/ml and a significant (p ≤ 0.05) decrease in the viability of these cells at the concentrations of 125, 62, and 31 μg/ml as compared to the control group. The ethanolic extract caused a statistically significant (p ≤ 0.001) increase in the viability of PBLs at the concentration 125 μg/ml and above as compared to the control group (Figure 2).
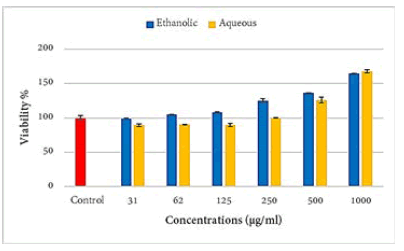
Figure 2: Effects of propolis extracts on the isolated PBL
Immunomodulatory effects of propolis on the Raji cells and isolated peripheral blood lymphocytes
The results exhibited a highly significant (P ≤ 0.001) decrease in IL-4 levels in the aqueous extract at concentrations 1000, 250, and 50 μg/ml and ethanolic extract at concentration 1000 μg/ml when compared to the control group (Figure 3).
The results also revealed a highly significant (P ≤ 0.001) increase in IL-4 level in the aqueous extract at concentrations 50 and 250 μg/ml, and a significant (p ≤ 0.05) in the ethanolic extract of propolis, while there was a highly significant (P ≤ 0.001) decrease in IL-4 level in ethanolic extract of propolis at concentration 1000 μg/ml when compared to the control group (Figure 3).
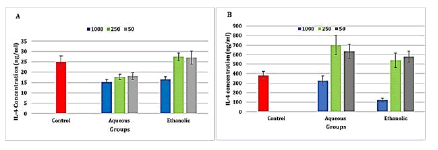
Figure 3: Level of interleukin 4 in the propolis extracts treated A-Raji cell line and B- isolated peripheral blood lymphocytes
There was a highly significant (P ≤ 0.001) increase in IL-10 levels in the ethanolic extract at concentration 50 μg/ml, while there was a highly significant (P ≤ 0.001) decrease in IL-10 levels in the ethanolic extract at concentration 1000 μg/ml when compared to the control group (Figure 4). The results showed a highly significant (P ≤ 0.001) increase in IL-10 levels in the aqueous extract at concentration 250 μg/ ml and a significant (p ≤ 0.05) increase in the ethanolic extracts at concentration 250 μg/ml when compared to the control group (Figure 4).
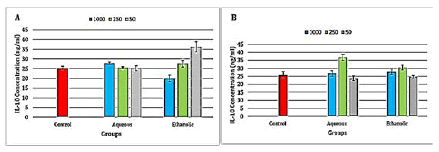
Figure 4: Level of interleukin 10 in the propolis extracts treated A-Raji cell line and B- isolated peripheral blood lymphocytes
Aqueous and ethanolic extracts of propolis caused a highly significant (P ≤ 0.001) decrease in IL-17 level in both at all concentrations (Figure 5).
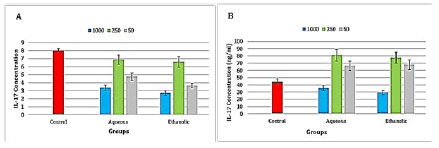
Figure 5: Level of interleukin 17 in the propolis treated A-Raji cell line and B- peripheral blood lymphocytes
There was a highly significant (P ≤ 0.001) increase in IL-17 level in after the treatment with aqueous and ethanolic extracts at concentrations 50 and 250 μg/ml, while there was a significant (P ≤ 0.05) decrease in IL-17 levels in the ethanolic extract at concentration 1000 μg/ml, also the concentration 1000 μg/ml of aqueous extract of propolis increase the level of IL-17 but no significant difference was found when compared to the control group (Figure 5).
A highly significant (P ≤ 0.001) decrease in INF-γ level was observed after the treatment with the aqueous extract at concentrations 50 and 1000 μg/ml and in the ethanolic extract at concentration 1000 μg/ml, while there was a significant (p ≤ 0.05) decrease in the level of INF-γ in the aqueous extract at concentration 250 μg/ml and ethanolic extract at concentrations 50 and 250 μg/ml (Figure 6).
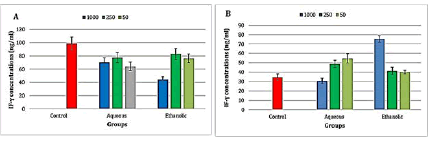
Figure 6: Level of interferon-gamma in the propolis-treated A-Raji cell line and B- peripheral blood lymphocytes
There was highly significant (P ≤ 0.001) increase in INF-γ levels in the aqueous extract of propolis at concentrations 50 and 250 μg/ml and the ethanolic extract at a concentration of 1000 μg/ml when compared to the control group (Figure 6).
Discussion
In the present study the aqueous extract of propolis at concentrations 1000, 500, and 250 μg/ml caused a significant decrease in the viability of the Raji cell line, this result is consistent with the result found by Spiridonov study in which propolis aqueous extracts cause complete suppressing to the growth of the Raji cell line after treatment with the concentrations ranging from 50-500 μg/ml. In the present study, ethanolic extract at the concentration 1000 μg/ ml caused a significant decrease in the viability of the Raji cell line and this effect may be attributed to artepillin C which has been found in the ethanolic extract of propolis and has antitumor activity on different human leukemia cell lines such as lymphocytic leukemia (both T-cell and B-cell lines), myeloid leukemia and it induces apoptotic cell death (marked by the appearance of apoptotic bodies and DNA fragmentation) in all the tested cells; and it had been suggested that this effect may be partly associated with up regulation of Fas expression and loss of mitochondrial membrane potential. In addition, the ability of propolis in decreasing the viability of the Raji cell line may be related to the presence of genistein and isoflavone that had been present in aqueous and ethanolic extracts of propolis. Also, the result of the current study is in agreement with the study of Campos et al. which reported that ethanolic extract of propolis was capable of reducing 50% of the K562 (leukemia) tumor cells growth [7].
The ethanolic extract of propolis at concentrations 1000, 500, 250, and 125 μg/ml caused a significant increase in the proliferation of lymphocytes, this result agrees with Arslan et al study which pointed out that the ethanolic extract of propolis enhanced human peripheral lymphocytes viability at concentrations 500, 250, 125, and 62.5 μg/ml and protect the cells against mitomycin cytotoxicity [8]. The proliferation of peripheral lymphocytes induced by propolis may be correlated to the flavonoids content, propolis’ flavonoids are difficult to dissolve in water [9], thus this fact could explain the low influence of low concentrations of propolis’ aqueous extract on the proliferation of lymphocytes reported by the present study. At higher concentrations (1000 and 500 μg/ml) of propolis’ aqueous extract, the significant increase in the viability of lymphocytes agrees with the result of the study performed by Mojarab et al. which indicated that the use of the aqueous extract of propolis as an adjuvant in Human Immunodeficiency Virus (HIV) vaccine increasing the proliferation of lymphocytes in mice treated with this formulation [10].
Propolis has a direct regulatory effect on the basic functional properties of immune cells, suppressing the release of pro-inflammatory cytokines and stimulating the production of anti-inflammatory cytokines like IL-4 [11]. Caffeic acid phenethyl ester (CAPE) increases the production of anti-inflammatory cytokines mainly IL-4 [12]. In the present study, the high significant decrease in the level of IL-4 after the treatment of the Raji cell line with aqueous (50, 250, and 1000 μg/ml) and ethanolic (1000 μg/ml) extracts of propolis goes with that found by Szliszka et al. study which pointed that the level of IL-4 in RAW264.7 cells monocyte/macrophage-like cells, originating from Abelson leukemia virus transformed cell lines derived from mice was reduced by artepillin C [13,14]. Also it goes with Szliszka study which observed that the level of IL-4 in J774A.1 cells (Reticulum cell sarcoma) was also reduced when treated with ethanolic extract of propolis [15]. In the present study, the increment in the level of IL-4 after the treatment of isolated PBLs with 50 and 250 μg/ml of both aqueous and ethanolic extracts of propolis is in agreement with Magnavacca et al. in vivo study which found that the subcutaneous administration of liposomal preparation of Chinese propolis flavonoids with ovalbumin to mice could effectively activate the cellular and humoral immune response, increasing the level of IL‐4 in the serum [16]. Regarding the decrease in the level of IL-4 after the treatment of PBLs with high concentration (1000 μg/ml) of propolis’ ethanolic extract reported by the current study it is consistent with the result of Chan et al. study which indicated that propolis suppresses cytokines production from Th2 such as IL-4, and this can be explained by the presence of chrysin and kaempferol which are flavonoids of propolis reported to inhibiting the release of cytokines from mast cells [17]. Also, Nakamura study pointed out that the administration of these flavonoids to mice results in the suppression of Th2 cytokines, such as IL-4 [18].
The decrement in the level of IL-10 after the treatment of the Raji cell line with 1000 μg/ml of ethanolic extract of propolis is in agreement with what has been reported by Szliszka et al. study which indicated that treatment of J774A.1 macrophage with ethanolic extract of propolis would cause slightly down-regulates in IL-10 production in culture supernatants derived from these cells. This may be attributed to the inhibitory effect of artepillin C on the production of IL-10 in vitro and in vivo models [19].
Regarding the increase in the level of IL-10 after the treatment of the Raji cell line with ethanolic (50 μg/ml) of propolis reported by the current study, it is in agreement with what has been found by Machado et al. in vivo study in which the mice with cotton pellet granuloma given propolis orally for six days increase IL-10 production [20]. Also, it agrees with Missima et al. in vivo study which pointed out that treatment of melanoma bearing mice with propolis for 14 days would induce IL-10 production [21].
Regarding the highly significant increase in the level of IL-10 after the treatment of PBLs with 250 μg/ml of both aqueous and ethanolic extracts of propolis that found by the current study, it is in agreement with that reported by Conti et al. who observed that the treatment of lymphocytes with either with Brazilian propolis (2, 10, and 20 μg/ ml) or Mexican propolis (1, 2, 10 and 20 μg/ml) exhibited a stimulatory effect in IL-10 production in a dose dependent manner [22]. This result may be related to the effect of CAPE as having been found by Conte in vivo study in which the CAPE was administered to Lipopolysaccharide- treated rats result in a decrease in the production of pro-inflammatory cytokines and an increase in the production of the anti-inflammatory cytokines such as IL-10 [22].
Regarding the high significant decrease in the level of IL-17 after the treatment of the Raji cell line with both aqueous and ethanolic extracts of propolis at all concentrations found by the current study, it is in agreement with that reported by Szliszka and her colleagues which pointed that the level of IL-17 in activated RAW264.7 cells was reduced by artepillin C [15]. Also the result of the current study is in agreement with that of Khosravi et al. in vivo study in which the mice with invasive ductal carcinoma given propolis orally for 10 days result in a decrease in IL-17 production [23]. While the result of the study made by Szliszka on J774A.1 macrophages is disagree with the result of the current study as it pointed out that the ethanolic extract of propolis did no influence on the level of IL-17 [15].
In the current study, the high significant decrease in the level of IL-17 after the treatment of PBLs with 1000 μg/ ml of propolis’ ethanolic extract is in agreement with the result of Cheung et al. who found that both artepillin C and ethanolic extract of propolis can significantly inhibit T cell proliferation, activation, and suppress the expression of IL-17 [24]. Moreover, a recent clinical study demonstrated that the level of IL-17 released from PBLs in HIV patients taking propolis at a dose of 500 mg/day for 3 months was significantly reduced [25]. The significant decrease in the level of IL-17 may be attributed to the inhibitory effect of high concentration (1000 μg/ml) of propolis ethanolic extract on Th2 cells as had been found by a recent study in which the ethanolic extract of Iranian propolis inhibits the release of IL‐17 induced by Aspergillus fumigatus conidia in murine lung epithelial cells [16]. Another in vivo previous study confirm the result of the current study as it examined the effect of propolis ethanolic extract on the production of IL-17 from splenocytes of normal mice and it observed concentration dependent declines in the production of IL-17 [26]. Regarding the highly significant increase in the level of IL-17 after the treatment of PBLs with low concentrations (250 and 50 μg/ml) of both aqueous and ethanolic extracts of propolis found by the current study it is in agreement with a recent in vivo study performed by Mohammed et al. which indicated that the oral administration of propolis (30, 40, and 50 mg/kg) to mice with wound infected with Acinetobacter baumannii would cause an increment in the level of IL-17 [27].
In the current study, the treatment of the Raji cell line with aqueous and alcoholic extracts of propolis significantly decreased the level of IFN-γ in all propolis concentrations; this result explains the anti-inflammatory and antitumor effects of propolis as it causes a decrease in the level of IFN-γ which acts as a proinflammatory cytokine in immune responses [28]. In this study, the reduction in the level of IFN-γ after the treatment of Raji cell line with propolis extracts may be attributed to artepillin C as have been reported by a recent study which revealed that artepillin C can inhibit IFN-γ and affects various immune cells, such as the suppression of T lymphocytes [29]. The exact mechanisms of these actions remain unclear till now [30]. In the current study, there was a highly significant increase in the level of IFN-γ in the PBLs treated with aqueous (250 and 50 μg/ml) and ethanolic (1000 μg/ml) extracts of propolis, this result is in agreement with a study done by Magnavacca which demonstrated that the subcutaneous administration of liposomal preparation of Chinese propolis flavonoids with ovalbumin to mice could efficiently stimulate the cellular and humoral immune response and increase the level of IFN‐γ in the serum [16]. Another in vivo study done by Sá-nunes et al. revealed that the production of IFN‐γ in the supernatant of spleen cell culture taken from propolis treated mice (mice were intraperitoneally treated with 2.5, 5, and 10 mg/ kg of propolis hydroalcoholic solution daily for 3 days) was increased.
Conclusion
Propolis promotes PBLs proliferation and had an antiproliferative effect against the Raji cell line. It had a dose dependent immunomodulatory effect.
Acknowledgement
None.
Conflict of Interest
Authors declare that there are no conflicts of interest.
References
- M. Ishtiaq, A. Ullah, K. Ali, M. Attaullah, H. Khan, Composition and functional properties of propolis ( bee glue ): A review, Saudi J Biol Sci, 26(2019), 1695-1703.
- M. Kumari, (2020), Cancer notes.
- X. Guan, Cancer metastases: Challenges and opportunities, Acta Pharm Sin B, 5(2015), 402-418.
- W.W.W.R.N. Org, B.W. Lockwood, Leukemia: AML, CML, ALL and CLL. CML.
- D. Li, C. Li, Y. Song, M. Zhou, X. Sun, et al. Marsdenia tenacssima extract and its functional components inhibits proliferation and induces apoptosis of human Burkitt leukemia/lymphoma cells in vitro and in vivo, Leuk Lymphoma, 57(2016), 419–428.
- F.A. Alwaeely, K.N. Madlum, M.A. Alsaadi, Immunomodulatory effect of propolis on Foxp3 gene expression in human peripheral blood mononuclear cells stimulated in vitro with pseudomonas aeruginosa ag, Arch Razi Inst, 76(4), 821–828.
- J.F. Campos, U.P. dos Santos, L.F.B. Macorini, A.M.M.F. de Melo, J.B.P. Balestieri, et al. Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae), Food Chem Toxicol, 65(2014), 374–380.
- M. Arslan, Y. Sevgiler, C. Güven, Z.T. Murathan, N. Erbil, Mellifera caucasica from the Ardahan and Erzurum provinces of Turkey : A comparative study, Arh Hig Rada Toksikol, 17(2021), 53–69.
- Y. Tao, D. Wang, Y. Hu, Y. Huang, Y. Yu, et al. The Immunological Enhancement Activity of Propolis Flavonoids Liposome In Vitro and In Vivo, Evid Based Complement Alternat Med, 2014(2014), 483513.
- S. Mojarab, D. Shahbazzadeh, M. Moghbeli, Y. Eshraghi, K.P. Bagheri, et al. Immune responses to HIV-1 polytope vaccine candidate formulated in aqueous and alcoholic extracts of Propolis: Comparable immune responses to Alum and Freund adjuvants, Microb Pathog, 140(2020), 103932.
- S.E.S. Elswefy, F.R. Abdallah, A.S. Wahba, R.A. Hasan, H.H. Atteia, Antifibrotic effect of curcumin, N-acetyl cysteine and propolis extract against bisphenol A-induced hepatotoxicity in rats: Prophylaxis versus co-treatment, Life Sci, 260(2020), 118245.
- N. Pahlavani, M. Malekahmadi, S. Firouzi, D. Rostami, A. Sedaghat, et al. Molecular and cellular mechanisms of the effects of Propolis in inflammation, oxidative stress and glycemic control in chronic diseases, Nutr Metabol, 17(2020), 1–12.
- E. Szliszka, A.Z. Kucharska, A. Sokół-ŁȨtowska, A. Mertas, Z.P. Czuba, et al. Chemical composition and anti-inflammatory effect of ethanolic extract of Brazilian green propolis on activated J774A.1 macrophages, Evid Based Complement Alternat Med, 2013(2013), 976415.
- B. Taciak, M. Białasek, A. Braniewska, Z. Sas, P. Sawicka, et al. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages, PLoS ONE, 13(2018), 1–13.
- E. Szliszka, A. Mertas, Z.P. Czuba, W. Król, Inhibition of inflammatory response by artepillin c in activated raw264.7 macrophages, Evid Based Complement Alternat Med, 2013(2013), 735176.
- A. Magnavacca, E. Sangiovanni, G. Racagni, M. Dell’Agli, The antiviral and immunomodulatory activities of propolis: An update and future perspectives for respiratory diseases, Med Res Rev, 42(2022), 897-945,
- G.C. Chan, K. Cheung, D.M. Sze, The immunomodulatory and anticancer properties of propolis, Clin Rev Allergy Immunol, 44(2013), 262-273.
- R. Nakamura, R. Nakamura, K. Watanabe, K. Oka, S. Ohta, et al. Effects of propolis from different areas on mast cell degranulation and identification of the effective components in propolis, Int Immunopharmacol, 10(2010), 1107–1112.
- B. Basak, R. Emran, P. Rozario, D. Obanda, Artepillin C: A comprehensive review of its chemistry, bioavailability, and pharmacological properties, Fitoterapia, 147(2020), 104775.
- J.L. MacHado, A.K.M. Assunção, M.C.P. Da Silva, A.S. Reis, G.C. Costa, et al. Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity, Evid Based Complement Alternat Med, 2012(2012), 157652.
- F. Missima, A.C. Pagliarone, C.L. Orsatti, J.P. Araújo, J.M. Sforcin, The Effect of propolis on Th1/Th2 cytokine expression and production by melanoma-bearing mice submitted to stress, Phytother Res, 24(2010), 1501–1507.
- B.J. Conti, K.B. Santiago, M.C. Búfalo, Y.F. Herrera, E. Alday, et al. Modulatory effects of propolis samples from Latin America (Brazil, Cuba and Mexico) on cytokine production by human monocytes, J Pharm Pharmacol, 67(2015), 1431–1438.
- A.R. Khosravi, H. Shokri, S. Darvishi, M. Taghavi, Immunomodulatory efficacy of ethanol extract of propolis on tumor-bearing mice with disseminated candidiasis, J Mycol Med, 24(2014), e143–e148.
- K.W. Cheung, D.M.Y. Sze, W.K. Chan, R.X. Deng, W. Tu, et al. Brazilian green propolis and its constituent, Artepillin C inhibits allogeneic activated human CD4 T cells expansion and activation, J Ethnopharmacol, 138(2011), 463–471.
- F.L. Conte, K.I. Tasca, K.B. Santiago, C.E. de Oliveira, G.G. Romagnoli, et al. Propolis increases Foxp3 expression and lymphocyte proliferation in HIV-infected people: A randomized, double blind, parallel-group and placebo-controlled study, Biomed Pharmacother, 142(2021), 111984.
- M. Tanaka, Y. Okamoto, T. Fukui, T. Masuzawa, Suppression of interleukin 17 production by Brazilian propolis in mice with collagen-induced arthritis, Inflammopharmacology, 20(2012), 19–26.
- R.I. Mohammed, A.Y. Alnuaman, M. Abd-aljabbar, The Effect Of Propolis On Level Of Interleukin 17 And Interleukin 37 In Experimentally Infected Mice With Acinetobacter Baumannii, Nat Volatiles Essent Oils, 8(2021), 2355–2365.
- S.H. Lee, J.Y. Kwon, S.Y. Kim, K.A. Jung, M.La. Cho, Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis, Sci Rep, 7(2017), 10133.
- F.A. Alwaeely, K.N. Madlum, M.A. Alsaadi, Immunomodulatory Effect of Propolis on IFN-γ, IL-17A and IL- 23 Production in Human Peripheral Blood Mononuclear Cells Treated with Pseudomonas aeruginosa Ag, J Chem Healt Risk, 11(2021), 821–828.
- F.P. Beserra, L. Fernando, S. Gushiken, V.P. Ribeiro, F. Bonamin, et al. Artepillin C as an outstanding phenolic compound of Brazilian green propolis for disease treatment : A review on pharmacological aspects, Phytother Res, 35(2020), 1–13.
Copyright: © 2022 Kaiser N. Madlum, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

