Research Article - Journal of Drug and Alcohol Research ( 2022) Volume 11, Issue 7
Development of a Novel Stability quoting RP-Ultra Performance Liquid Chromatography approach for Synchronous Assessment of Doravirine, Lamivudine, and Tenofovir Disproxil fumarate in Pure API Form and Tablet Dosage Based on ICH Guidelines
Challamalla Pavani and V. Jayashree*V. Jayashree, Department of Pharmaceutical Analysis, Vels Institute of Science, India, Email: Jayashree@yahoo.co.in
Received: 04-Jul-2022, Manuscript No. jdar-22-70166; Editor assigned: 06-Jul-2022, Pre QC No. jdar- 22-70166(PQ); Reviewed: 20-Jul-2022, QC No. jdar-22-70166; Revised: 25-Jul-2022, Manuscript No. jdar-22- 70166 (R); Published: 01-Aug-2022, DOI: 10.4303/jdar/236188
Abstract
The concomitant measurement of Tenofovir DF, Lamivudine, and Doravirine in bulk and tablet dose form with UPLC is the subject of this study. The separation was performed using acetonitrile and 0.1% TEA buffer at pH-3 as the mobile phase on an Endoversil, C18, ODS (2.1 50 mm 1.7 m) analytical column. The eluents were identified at 260.0 nm using a PDA detector. Doravirine, Lamivudine, and Tenofovir DF were separated at 0.805, 0.326, and 0.481 min, correspondingly, under ideal conditions. Lamivudine had a 0.09 μg/mL of detection limit, Tenofovir DF had a detection limit of 0.04 μg/mL, and Doravirine had a detection limit of 0.09 μg/mL. Lamivudine had a percentage mean recovery of 99.68 percent, Tenofovir DF had a percentage mean recovery of 99.55 percent, and Doravirine had a percentage mean recovery of 100.17%. In all of the stressful settings, the percentage of deterioration was found at a very low extent. Optimized conditions were discovered to be exceptionally suitable for determining all of them concurrently in both marketed dose form and bulk form.
Keywords
UPLC; Method development; Validation; Lamivudine; Tenofovir DF; Doravirine
Introduction
Lamivudine is an antiretroviral drug that is used to cure hepatitis B and HIV-1 infections. It operates by interfering with the synthesis of viral DNA. Chemical name of Lamivudine is 4-aminolamivudine. “-1-[(2R, 5S)-2-(hydroxymethyl)- 1, 3-oxathiolan-5-yl] pyrimidin-2-one dihydropyrimidin- 2-one dihydropyrimidin-2-one dihydropyr” (1, 2-dihydropyrimidin-2-one) [1]. Gilead Sciences sells the tenofovir prodrug disoproxil fumarate under the trade name Viread. It belongs to the nucleotide analog reverse transcriptase inhibitors family of antiretroviral medicines. Hepatitis B and HIV may be treated with this drug in combination with other medicines [2]. Doravirine is a non-nucleoside reverse transcriptase inhibitor therapeutic option for HIV-1 (“Human immunodeficiency virus type 1”) virus in adult individuals who have never undergone antiretroviral treatment, increasing the treatment choices for HIV-1 [3,4]. DELSTRIGO, a Doravirine, Lamivudine, and Tenofovir Disoproxil Fumarate combination, was approved by the FDA in August 2018. It’s designed to be used as a total treatment for HIV-1 infection in individuals who have never had antiretroviral medication before [5]. A thorough review of the literature finds that no UV spectrophotometric methods have been described; however, LC-MS/MS bioanalytical methods are available. There was no mention of the combination of these three antivirals in any pharmacopeia. In the literature, there were only a few HPLC and a single UPLC method that could simultaneously estimate the current combination [6-9]. All of the mentioned HPLC methods had longer retention times and had additional sensitivity issues, such as Kokkirela et al., Tenofovir DF was eluted in less than four minutes using acetonitrile/ phosphate buffer as the mobile phase. According to the ICH criteria, Gowri et al. found up to 33.8 percent degradation using n-hexane as a mobile phase. To elute Doravirine, an HPLC procedure described by Tiruveedhi et al. took as much as 8.16 minutes to complete. Even though all of the analytes’ quantitation limits were high, there were some sensitivity concerns. The only method disclosed was Addanki et al. UPLC method, the mobile phase was potassium and acetonitrile dihydrogen phosphate buffer, which was pertinent to the present inquiry. Doravirine eluted in 1.25 minutes, Lamivudine in 1.498 minutes, whereas Tenofovir DF in 1.823 minutes. These retention periods can be further decreased to less than one minute by utilizing ultra- performance chromatography. The stated sensitivities of all analytes in terms of quantitation and detection limits may be improved. It’s possible to use ultra-performance liquid chromatography to analyse various classes of active pharmaceutical ingredients because of the advantages of this technique over more traditional HPLC in terms of process reliability, turnaround time, the sensitivity of the method, and drug specificity [10]. By enhancing sensitivity and shortening elution periods, Tenofovir DF Lamivudine, and Doravirine in bulk pharmaceuticals and commercial dose concentration are the focus of the present research effort to overcome possible constraints and create a stability indicating ultrafast UPLC approach. ICH (“International Conference on Harmonization”) Q2 (R1) criteria were also followed in conducting the validation research [11].
Materials and Methods
Experimental
Chemicals and instruments: WATERS Acquity UPLC using Empower 2 software and a 2996 PDA detector. LABINDIA UV 3000+ UV/VIS spectrophotometer, Afcoset ER-200A weighing balance, and Adwa – AD 1020 pH meter were used in the investigation. Merck, Mumbai, India, provided HPLC water, acetonitrile, and orthophosphoric acid.
Pure samples and formulation: Hetero laboratories in Hyderabad, India, provided the pharmaceutical components Lamivudine, Tenofovir DF, and Doravirine with purity levels of 99.43%, 99.71%, and 99.41%. A local pharmacy in Hyderabad, India, sold commercially available medicines.
0.1 percent TEA buffer preparation: After diluting one milliliter of Triethylamine in a volumetric flask of 1000 mL with OPA, pH was adjusted to 3. The fluid was then sonicated for 10 minutes after passing through a 0.45-micron membrane filter.
Mobile phase preparation: 40 ml of the aforementioned buffer (40%) and 600 ml of Acetonitrile UPLC (60%) were precisely measured and dissolved in an ultrasonic water bath for 10 min before being vacuum filtered using a 0.45 filter.
Diluent: The diluting agent was the mobile phase.
Preparation of the working standard solution: Weigh and transfer into a 100 mL clean dry volumetric flask, mix 300 mg Lamivudine, 300 mg Tenofovir, and 100 mg Doravirine. Then add around 70 mL of Diluent and sonicate to thoroughly dissolve, then add the equivalent amount of solvent to make up the rest of the volume (Stock solution). Fill a 10 mL volumetric flask halfway with 0.6 mL of the abovementioned stock solutions and dilute with diluent to the desired concentration.
Sample solution preparation: Weigh 10 tablets and crush them with a motor and pestle to produce Lamivudine of 300 mg, Tenofovir of 300 mg, and Doravirine tablet powder of 100 mg (i.e., 1077 mg Avg wt. consumed 1085 mg) in a 10 mL clean dry volumetric flask. It took 30 minutes of sonication to thoroughly dissolve the content in the 70 mL of Diluent, which was then added to the final volume. 0.6 ml of stock solution was poured into a 10 ml volumetric flask and dilution with diluent until the necessary concentration was attained, followed by filtration through a 0.45-micron Injection filter (Stock solution). 2 ml of the aforementioned solution was used to incubate two plates with three different concentrations of Lamivudine, Tenofovir, and Doravirine to determine the percent Assay.
Validation of the RP-UPLC technique
System suitability study: To determine whether an analytical system was performing correctly was conducted. The standard drugs: Lamivudine (1.8 mg/mL), Doravirine (0.6 mg/mL), and Tenofovir DF (1.8 mg/mL) were used to test it six times. Several characteristics were considered for determining the percentage relative standard, including the theoretical plate, retention period, and asymmetry factor.
Accuracy study: The accuracy of the newly created approach was investigated using a recovery study. Fixed-dosage tablet powder combination was spiked with standard samples of Lamivudine, Tenofovir, and Doravirine at concentrations of 50%, 100%, and 150%, respectively. Calculations were made to determine the total quantity of each dosage yield. % RSD was measured for each level, and total recovery for all medicines was analysed. There should be 98.0%-102.0% recovery at each step.
Precision study: Lamivudine (1.8 mg/mL), Doravirine (0.6 mg/mL), and Tenofovir DF (1.8 mg/mL) were added to the main standard stock solution for the precision investigation of the proposed approach. There have been 6 injections of this prepared solution and areas were measured utilizing a UPLC. RSD % was discovered to be within specified limits for six replicate injections area. RSD% for the result area from 6 standard injections shouldn’t be over 2 percent.
Intermediate precision
On the other day, a spiked solution comprising all four APIs was produced similarly to the precision study, also injected 6 times, each injection area being determined in the UPLC. RSD % for results area from 6 standard injections shouldn’t be over 2 percent.
Linearity
To test the linearity of the present technique, dilutions of the standard solution of Lamivudine, Tenofovir DF, and Doravirine were made. The linearity investigation used different dosages of Lamivudine 60-300 μg/mL, Tenofovir DF 60-300 μg/mL, and Doravirine 20-100 μg/mL. All these solutions had their calibration curves drawn at various levels. After constructing a linearity graph based on peak and concentration area, the data were analysed using regression.
Limit of detection study (LOD)
To study the limit of detection, a working standard solution containing 300 mg of Tenofovir DF, 300 mg of Lamivudine, and 100 mg of Doravirine was carefully weighted as well as diluted as reported before. To prepare 0.09 μg/mL of Tenofovir DF and Lamivudine, a further dilution was made from the aforementioned solution by extracting an appropriate amount of aliquote volume. Doravirine had a final concentration of 0.04 μg/mL. Baseline noise and signal noise have been considered to compute S/N ratio, which must be 3.
Limit of quantitation (LOQ) study
The working standard solution provided previously in this section was used to make sample solutions for the quantitation limit experiment. We then prepared solutions of Tenofovir 0.30 μg/mL, Lamivudine 0.30 μg/mL, and Doravirine 0.13 μg/mL by diluting suitable volumes of the standard solution in a 10 mL volumetric flask with the diluent. The chromatographic equipment was used to analyze the prepared solutions. Baseline noise and signal noise have been considered to compute S/N ratio, which must be 10.
Robustness study
In the robustness testing of the newly created technique, the organic content of mobile phase and detection wavelength of optimal condition was purposefully modified by 10%. It was decided to increase the flow velocity to 0.3, the organic content to 10 percent, whereas 2 nm of detecting wavelength. The peak areas in all of these conditions determined the resilience of all of the APIs studied. Calculated % RSD of the parameters, which shouldn’t be more than 2.
Forced degradation study
ICH-recommended stress conditions were applied to the present sample solution [12], including oxidative, acidic, thermal, alkaline, and photolytic stress. The mean peak area was taken into account when calculating the results of all degradation tests, which were performed three times.
Degradation in an acidic environment
When the stock solution was poured into a volumetric flask, it was pipetted into the flask with 3 mL of 0.1 N HCl. Diluent was mixed into the flask to get the volume up to 10 mL after 6 hours of heating at 60°C. The solution was mixed into vials after filtration using 0.22-micron syringe filters.
Degradation in an alkaline environment
2 mL of the above stock solution and 3 mL of 0.1 N HCl was poured into a 10 mL volumetric flask. Diluent was added to the 0.1 N NaOH volumetric flask to get the volume up to 10 mL after 6 hours of heating at 60°C. The solution was mixed into vials after filtration using 0.22-micron syringe filters.
Degradation caused by heat
Lamivudine, Doravirine, and Tenofovir DF samples have been put in a petri dish, and also baked for 24 hours at 1100 degrees Celsius in a hot air oven. After being diluted with diluents, the substance was inserted into HPLC and analyzed.
Degradation by oxidation
It was done by adding 2 mL of the stock solution stated above and 1 mL of 3% w/v hydrogen peroxide to a 10 mL volumetric flask and then filling the flask to the mark with diluent. At room temperature for 15 minutes thereafter, the volumetric flask kept resting. The solution was mixed intovials after filtration using 0.45-micron syringe filters.
Degradation by photolysis
To degrade the sample solution of Lamivudine, Tenofovir DF, and Doravirine, UV light exposure was used for 15 minutes to seven days. Photolytic degradation, as stated above, results in the drug degradation seen.
Results and Discussion
Method optimization
Preliminary trials were conducted to the point where several parameters of the new technique may be optimized. In varied volume ratios, methanol, acetonitrile, and pH-varying buffers were used. Chromatographic characteristics such as peak form, retention duration, and resolution, as well as others were optimized by varying mobile phase flow rates with respect to the changing column temperatures. Acetonitrile buffered at pH-3 was the final mobile phase that worked well for all four APIs, providing great resolution and peak morphology. A C18, ODS, (2.1 50 mm 1.7 m) analytical column is considered to be suitable for elution with adequate resolution at a flow rate of 1 mL/min and a 260 nm wavelength of PDA detector. Lamivudine eluted at 0.326 minutes, Tenofovir DF (0.481 minutes), and Doravirine (0.805 minutes) were measured under ideal conditions. (Figures 1 and 2) shows the best chromatogram for each studied API. Throughout the validation parameter analysis, this optimum state has been maintained.
Figure 1: Chemical structures of Lamivudine (A), Tenofovir Disoproxil Fumarate (B), Doravirine(C).
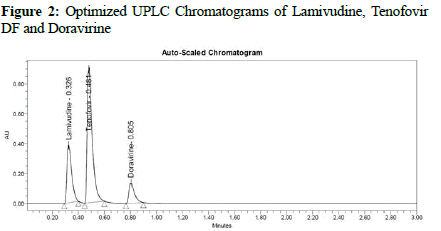
Figure 2: Optimized UPLC Chromatograms of Lamivudine, Tenofovir DF and Doravirine
Lamivudine, Tenofovir DF, and Doravirine were assayed following and the corresponding chromatogram (Figures 3-5), and % assay was found to be 100.74%, 100.78%, and 100.43% for Lamivudine, Tenofovir DF, and Doravirine, correspondingly (Table 1). Testing on the marketed dose forms of Lamivudine, Tenofovir DF, and Doravirine produced findings that closely matched the specified label and are within admissible limits, suggesting that the proposed approach can analyze all three APIs in the marketing dose form concurrently. According to the accuracy of research results, the mean % RSD for Tenofovir DF, Lamivudine, and Doravirine was 99.55%, 99.68%, and 100.17%, correspondingly. As a consequence, according to the guidelines, the accuracy of the current method was determined, and all analytes were determined to be recovered within the permitted limit of 98-102%, as illustrated by outcomes, validating the validity of the existing approach. The percent relative standard deviation for Lamivudine, Tenofovir DF, and Doravirine, respectively, was determined to be 1%, 0.6 percent, and 0.8 percent in the precision analysis of this approach. RSD% values for all tested parameters for all APIs were determined to be under 2 in the intermediate precision. Consequently, in the precision and moderate precision trials, the levels of all three factors were verified and RSD% was observed to be acceptable and within an admissible limit. According to the outcomes of the precision investigation, the precision of the current technique for measuring all analytes simultaneously in bulk and their marketing pharmaceutical formulations was evaluated. To confirm that the UPLC equipment used for analytical measures was operating properly, a system appropriateness study was done. Among the factors studied were peak area, tailing factor, and theoretical plates. For Lamividine, Tenofovir DF, and Doravirine, the % RSD of tailing factors, peak area, retention duration, and theoretical plates was 1.39%, 0.23%, 1.41%, and 0.21%, correspondingly (Table 2). All of the metrics tested were found to have a percent RSD of less than 2. The system suitability value shows that under ideal circumstances, the employed UPLC approach was considered acceptable for the examination of all analytes tested. In the linearity investigation, the linear range for Lamivudine at 60-300 μg/mL range, 60-300 μg/ mL for Tenofovir, and 20-100 μg/mL for Doravirine. The correlation coefficient for the complete active component was found to be 0.999 on the linearity graph. The linearity examination of the currently proposed technique indicated that the correlation coefficient for all four APIs was close to 1, which is within an admissible limit and shows linearity. The least squares technique was followed to create the regression line, and all API curves were shown to be linear. Lamivudine had a LOD of 0.09 μg/mL, Tenofovir DF had 0.04 μg/mL, and Doravirine had 0.09 μg/mL. The LOQ for Lamivudine was 0.30 μg/mL, 0.13 μg/mL for Tenofovir DF, and 0.29 μg/mL for Doravirine in LOQ trial. The sensitivity of the proposed approach was demonstrated by LOD and LOQ results for all analyzed APIs. When the flow rate, detecting wavelength, and chemical composition were all altered purposefully, % RSD of peak area was analyzed to compute robustness, yielding 0.29 μg/mL 1.38 μg/mL, and 1.67 μg/mL for Lamivudine, Tenofovir DF, and Doravirine correspondingly. The findings are given in detail in (Table 3). The results of the robustness study show that the present method is exceptionally resilient for all APIs with small variations in chromatographic parameters and elution APIs with their given retention duration and appropriate resolution. In the force degradation investigation, Lamivudine, Tenofovir DF, and Doravirine all demonstrated 7.34% degradation in an acidic stressed environment, 5.03%, and 3.40%, respectively. Almost the majority of the stressful situations revealed similar degradation trends for all three analytes tested. During the severe degradation investigations, the purity threshold as well as peak purity angle was also discovered. (Table 4) illustrates that the % degradations were found at a very low level and were determined to be within the rules’ allowed standards. Procedure stability has been shown by purity threshold as well as purity angle being within admissible limits. The required chromatograms for all four APIs, and the degraded peaks, were eluted with adequate resolution in their fixed areas.
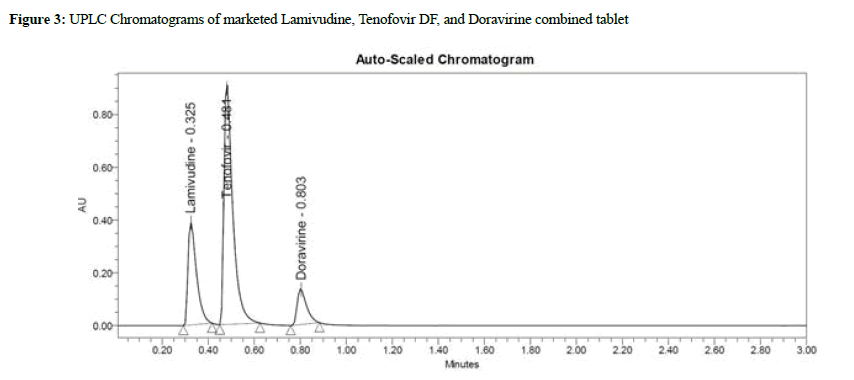
Figure 3: UPLC Chromatograms of marketed Lamivudine, Tenofovir DF, and Doravirine combined tablet
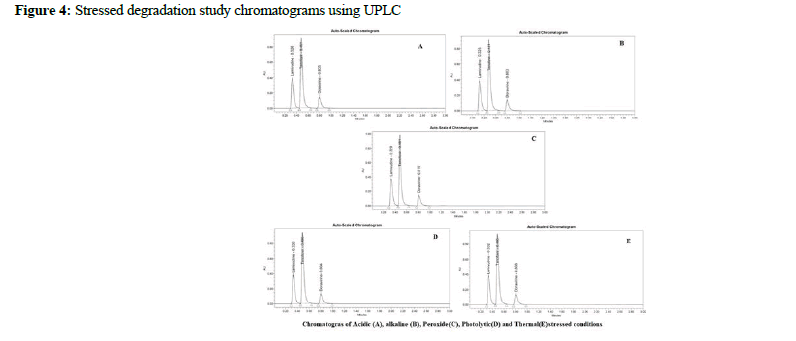
Figure 4: Stressed degradation study chromatograms using UPLC
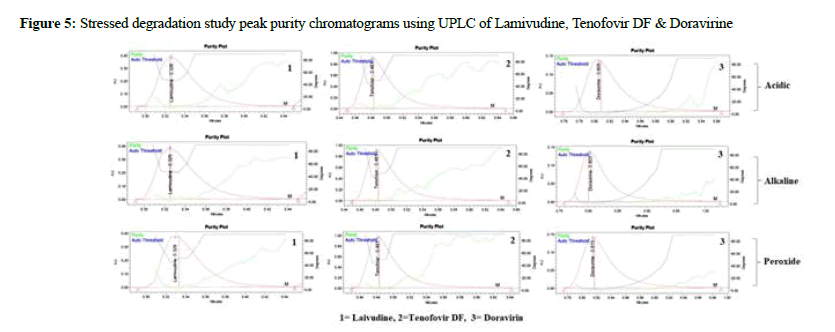
Figure 5: Stressed degradation study peak purity chromatograms using UPLC of Lamivudine, Tenofovir DF & Doravirine
| Formulation | Lamivudine | Tenofovir-DF | Doravirine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Obtained amount (mg) | Obtained amount (mg) | % Assay* |
Amount taken (mg) |
Amount obtained (mg) |
% Assay* |
Amount taken (mg) |
Amount obtained (mg) |
% Assay* |
|
| Delstrigo tablets (Lamivudine+Tenofovir+Doravirine) | 300 | 302.22 | 100.74 | 300 | 302.24 | 100.78 | 100 | 100.43 | 100.43 |
Note: *Three replicates on average
Table 1: Marketed formulation assays
| Parameters | Lamivudine | Tenofovir | Doravirine |
|---|---|---|---|
| Precision (%RSD) | 1.0 | 0.6 | 0.8 |
| Intermediate precision (%RSD) | 0.2 | 0.8 | 0.2 |
| Accuracy (Mean recovery) | 99.68 | 99.55 | 100.17 |
| Regression coefficient | 0.9997 | 0.9996 | 0.9995 |
| Linearity (µg/mL) | 60-300 | 60-300 | 20-100 |
| LOD (µg/mL) | 0.09 | 0.04 | 0.09 |
| LOQ (µg/mL) | 0.30 | 0.13 | 0.29 |
Table 2: Validation parameter results
| Parameters | Changes Done | Lamivudine | Tenofovir-DF | Doravirine |
|---|---|---|---|---|
| Variations in flow rate | Area* | Area* | Area* | |
| 0.27 mL | 971746 | 2368338 | 388976 | |
| 0.3 mL | 996045 | 2373750 | 399197 | |
| 0.33 mL | 990152 | 2405151 | 381173 | |
| Organic composition in mobile phase | 10 %less | 971746 | 2338338 | 388976 |
| Actual | 996045 | 2373750 | 399197 | |
| 10% more | 990152 | 2405151 | 381173 | |
| Detection wavelength | 258 nm | 971658 | 2405474 | 382145 |
| 260 nm | 990542 | 2406523 | 399652 | |
| 262 nm | 990241 | 2398545 | 385645 | |
| % RSD | - | 0.79 | 1.38 | 1.67 |
Note: *Average of three readings
Table 3: Robustness study
| Stressed conditions | Lamivudine | Tenofovir | Doravirine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % Degradation | Purity Angle | Purity Threshold | % Degradation | Purity Angle | Purity Threshold | % Degradation | Purity Angle | Purity-Threshold | |
| Alkaline | 6.58 | 15.97 | 90.00 | 4.95 | 15.04 | 90.00 | 3.27 | 0.75 | 4.37 |
| Acid | 7.34 | 11.88 | 90.00 | 5.02 | 15.31 | 90.00 | 3.40 | 0.76 | 4.08 |
| Peroxide | 7.15 | 16.86 | 90.00 | 4.86 | 13.03 | 90.00 | 3.07 | 0.65 | 4.07 |
| Photolytic | 7.12 | 14.61 | 90.00 | 4.66 | 13.36 | 90.00 | 3.12 | 0.76 | 4.33 |
| Thermal | 7.17 | 16.46 | 90.00 | 4.82 | 15.77 | 90.00 | 3.17 | 0.64 | 4.14 |
Table 4: Forced degradation study results
Conclusion
Based on empirical evidence, the simultaneous detection of Tenofovir DF, Lamivudine, and Doravirine using UPLC was found to be precisely similar. When compared to the previously reported ways, it was concluded that the new method was superior in every regard. Under ideal circumstances, all of the evaluated APIs were found to be relevant and resolute for simultaneous analysis in bulk form and authorized dosage form.
Acknowledgements
The authors would like to gratefully acknowledge all cooperation and support from the patients and hospital administration for facilitating this study.
Source of Funding
None.
Conflict of Interest
The authors have no conflicts of interest regarding this study.
References
- A. Luo, X. Jiang, H. Ren, Lamivudine plus tenofovir combination therapy versus lamivudine monotherapy for HBV/HIV coinfection: A meta-analysis, J Virol, 15(2018), 39.
- L. Williamson, N.A. Reynolds, G.L. Plosker, Tenofovir disoproxil fumarate: A review of its use in the management of HIV infection, J Drugs, 65(2005), 413–432.
- A.E. Rock, J. Lerner, M.E. Badowski, Doravirine and its potential in the treatment of HIV: An evidence-based review of the emerging data, HIV AIDS (Auckl), 12(2020), 201–210.
- M. A Colombier, J. M. Molina, Doravirine: A review, Curr Opin HIV AIDS, 13(2018), 308–314.
- C. Orkin, K.E Squires, J.M. Molina, P.E. Sax Sussmann, G. Lin, et al., Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate (TDF) Versus Efavirenz/Emtricitabine/TDF in treatment-naive adults with human immunodeficiency virus type 1 , Clin Infect Dis, 73(2021), 33-42.
- T.K. Kokkirala, P. Kancherla, P. Alegete, D. Suryakala, Stability indicating RP-HPLC method development and validation for the estimation of sofosbuvir, velpatasvir and voxilaprevir in bulk and pharmaceutical dosage form, Biomed Chromatogr, 35(2021), e5121.
- G. Gollu, S. Gummadi, A new stability indicating RP-UPLC method for simultaneous estimation of Doravirine, J Pharm Chem, 54(2020), 526–535.
- V.L. Tiruveedhi, V.R. Battula, K.B. Bonige, RP-HPLC (stability-indicating) based assay method for the simultaneous estimation of doravirine, tenofovir disoproxil fumarate and lamivudine, Int J Appl Pharmaceut, 13(2021), 153–159.
- S. Addanki, B.R. Kuber, A new stability indicating RP-UPLC method for simultaneous estimation of Doravirine, Lamivudine and Tenofovir disoproxil fumarate in bulk and their combined pharmaceutical formulation, Future J Pharm Sci, 7(2021), 221.
- P. Mondal, K. Mahender, B. Padmaja, A novel UPLC-PDA method for the simultaneous determination of lamivudine, zidovudine and nevirapine in bulk and tablet dosage form, Anal Chem Lett, 8(2018), 131-138.
- S. Mondal, S. Biswal, P. Senapati, P. Mondal, K. Bhar, et al., Furosemide-loaded alginate microspheres prepared by ionic cross-linking technique: Morphology and release characteristics, Int J Pharm Res, 13(2021) 124-133.
- P. Mondal, S. Shobharani, R. Ramakrishna, Development and validation of liquid chromatography method using the principles of QbD for antimalarials used in Artemisinin based combination therapy, Curr Pharm Anal, 10(2014), 271-278.
Copyright: © 2022 V. Jayashree, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

