Research Article: Journal of Drug and Alcohol Research (2023) Volume 12, Issue 5
Drug Development and Validation of a Sensitive Bio-analytical LCMS/MS Method for Quantification of Asciminib-a Chronic Myeloid Leukemia Drug in Human Plasma
Govindarao Yedlapalli1* and Y. Ganesh Kumar22Research Supervisor, Department of Pharmaceutics, Bir Tikendrajit University, India
Govindarao Yedlapalli, Research Scholar, Department of Pharmaceutical Analysis and Quality Assurance, Bir Tikendrajit University, India, Email: govind057@gmail.com
Received: 31-May-2023, Manuscript No. JDAR-23-103891; Editor assigned: 02-Jun-2023, Pre QC No. JDAR-23-103891 (PQ); Reviewed: 16-Jun-2023, QC No. JDAR-23-103891 ; Revised: 21-Jun-2023, Manuscript No. JDAR-23-103891 (R); Published: 28-Jun-2023, DOI: 10.4303/JDAR/236242
Abstract
This study targeted to develop a reliable and less time consuming Liquid Chromatography–Mass Spectrometry/Mass spectrometry (LC–MS/MS) method for quantification of chronic myeloid leukemia drug, asciminib in human plasma using sunitinib as internal standard. The analyte asciminib and sunitinib were extracted from spiked plasma by adopting liquid-liquid extraction using methyl tertiary butyl ether solvent and chromatographed on an Alltima HP C18 (100 mm × 4.6 mm, 3 μm) column with pH 4.3 ammonium formate (5 mM) buffer and methanol in 45 (v/v):55 (v/v) at pH 4.3 mL/min at 1.0 mL/min flow in isocratic mode. The analysis completed within a total chromatographic run time of 4 min that facilitates less time and solvent consumption. The column separated analytes were recorded using mass detector with positive ion electrospray ionization source. The mass spectrum shows precursor-to-product ion transitions at m/z of 450/195 (m+1) for asciminib and 399/185 (m+1) for sunitinib. The method produces calibration curve linear in 2.5 ng/mL-200 ng/mL concentration range with sensitive detection limit of 0.075 ng/mL for asciminib. The mean plasma spiked extraction recoveries of both asciminib and internal standard was very high with acceptable % RSD in all precision studies. The analytes were noticed to be stable in a variety of stability studies performed. The method was validated to be sensitive, accurate and was suitable for determination of asciminib in human plasma and applicable for regular quality analysis studies.
Keywords
Asciminib; LCMS/MS analysis; Bio-analytical method; Spiked human plasma; Stability studies
Introduction
The identification and quantitative analysis of minor quantity of analytes in biological samples such as tissues, plasma, serum, blood, urine, faces, saliva, hair etc., was considered as bioanalysis [1]. It plays significant role in drug development process by evaluating toxicological profile, pharmacokinetic and pharmacodynamics of drug [2]. Bioanalytical method development and validation plays crucial role in quantitative evaluation of not only small molecules like drugs and metabolites but also to quantify bigger molecules like peptides and proteins in biological samples. Bioanalytical methods were significantly useful in many research areas including development of novel drugs, doping control, forensic analysis, biomarkers identification for disease diagnosis [3].
Asciminib belongs to protein kinase inhibitor class medical drug prescribed to treat Philadelphia chromosome-positive chronic myeloid leukemia including T315I mutation [4]. The upper respiratory tract infections, fatigue, rash, nausea, musculoskeletal pain, and diarrhea are the possible side effects during the use of asciminib. The patient exhibiting abnormal blood tests, bone pain, cold, muscle and joint pain are also possible during the usage of asciminib [5]. It has 449.84 g/mol molecular mass with formula of C20H18ClF- 2N5O3 and its molecular structure was presented in Figure 1.
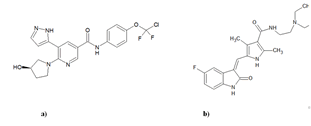
Figure 1: (a) structure of asciminib; (b) internal standard sunitinib
The literature survey was conducted to determine the methods available for quantification of asciminib. In literature, no analytical or bioanalytical method published for quantification of asciminib in samples. Keeping in view of the significance of the bioanalytical methods and lack of bioanalytical method for the quantification of asciminib, this study focused to optimize a sensitive and stable bioanalytical method for quantification of asciminib in human plasma. Another protein kinase inhibitor drug sunitinib was utilized as internal standard.
Materials and Methods
The asciminib standard drug with 98.67% purity and internal standard sunitinib (98.27% purity) was obtained from Novartis pharmaceuticals, Hyderabad, Telangana. The HPLC grade acetonitrile, methanol and milli Q water were procured from Merck chemicals, Mumbai. The local diagnostic laboratory provides the human healthy blood sample.
Equipment
The analysis was conducted from alliance-2695 (Waters, Japan) HPLC system equipped with triple quadrupole (Waters ZQ, Japan) mass detector, 0.1 μL–1500 μL injectable auto-injector and the chromatographic integrations was carried using masslynx 4.2 (Waters, Japan) software. Stuart™ scientific vortex mixer (SA8, Fisher Scientific, USA) was used for vortexing the analytes. The bench top centrifuge (C-852, Remi, India) was used for centrifugation of the analytes. Pasteur pipette was used for separating plasma from whole blood after centrifugation.
Standard solutions
The asciminib primary stock solutions for preparing standard calibration curve dilutions as well as Quality Control (QC) samples were prepared using independent weighing’s. The asciminib standard stock solution at 1 mg/mL was prepared using methanol solvent and the prepared stock solution was preserved in a refrigerator at 2°C-8°C. The working standard dilutions of asciminib were prepared using appropriate dilutions from standard stock solution using methanol solvent. The sunitinib working solution at 100 μg/mL concentration was prepared using methanol solvent and then refrigerated.
Calibration curve standards and quality control samples
The calibration dilutions were arranged by adding 5 mL of selected concentration of analytes to 9.5 mL human plasma. The calibration range consist of 8 non-zero concentrations in the level of 2.5 ng/mL to 200 ng/mL of asciminib and 100 ng/mL of sunitinib. Precision and accuracy samples was set up by spiking appropriate concentration of asciminib and 100 ng/mL of sunitinib internal standard to blank plasma. The standard asciminib samples prepared were 2.5 ng/mL (LLOQ), 5 ng/mL (LQC), 50 ng/mL (MQC), and 200 ng/mL (HQC) and the all the QC levels were spiked with 100 ng/mL of sunitinib internal standard. The prepared QC samples were preserved at 6°C for further use.
Extraction protocol
In a vortex centrifuge containing 0.5 mL of control human plasma, 20 μL of selected concentration of asciminib and 20 μL of internal standard at 100 ng/mL concentration was added and vortex for 1 min. Then methyl tertiary butyl ether (4 mL) was added and again vortex for 3 min. The supernatant clear organic layer was separated using Pasteur pipette and dried with nitrogen stream. The dried extract was reconstituted 100 μL using suitable diluent (equal volume of methanol and acetonitrile).
Method development
The development of method for resolution and estimation of asciminib using sunitinib internal standard was carried by conducting the trails in various method conditions. The method conditions include composition, flow rate, pH of mobile phase, stationary phase configuration and its temperature. The mas operating conditions like Collision Energy (CE), Entrance Potential (EP), Cell Exit Potential (CEP) and Declustering Potential (DP) were optimized. In the process of method development, one parameter change by keeping other in constant and each studied condition, spiked standard solution containing known concentration of asciminib and internal standard was analysed. The results observed in each changed condition was summarized and conditions that produce acceptable results in terms of specificity, symmetry and system suitability was studied for validation.
Method validation
Bioanalytical method validation guidelines issued by FDA were adopted for validating the method optimized for quantification of asciminib [6].
Specificity and selectivity
The extraction of analytes was carried for the blank plasma matrix as well as the blank plasma spiked with LLOQ level concentration of asciminib and internal standard. A zero sample that was prepared by spiking internal standard only without analyte was also analysed. The extracted unspiked and spiked plasma samples were analysed and the results achieved were summarized for evaluating method specificity and selectivity.
Linearity and range
The method was intended to analyse asciminib in human plasma samples and hence the calibration curve dilutions were prepared in human plasma. The anticipated calibration range, peak area response relationship of asciminib and internal standard suggest the number of calibration dilutions prepared for constructing the calibration curve. The calibration curve solutions consists of a blank sample that was spiked with no analytes, zero sample that was prepared by spiking known concentration of internal standard and eight dilutions that covers the anticipated range includes LLOQ. The analysis range in the proposed method was assessed by performing linear regression analysis. Calibration plot was prepared by plotting peak area response ratio of asciminib and internal standard against the prepared asciminib strength.
Accuracy and precision
Precision and accuracy of the developed method was conducted in 6 analyses in each concentration and 4 concentration levels in linearity range. Accuracy was confirmed by evaluating the variation of mean response form the actual value.
The precision experiment was performed as within-run, intra- batch precision that was carried in single analytical run whereas the inter-batch precision or repeatability was conducted in 2 different analysts in 3 days. Ruggedness was evaluated by performing the precision by change in analysts. The coefficient of variation in precision study should not exceed 15% in each studied concentration level except LLOQ where in, it should not exceed 20%.
Recovery
The recovery of asciminib from the spiked plasma need not to be 100% but the recovery extent of asciminib and internal standard must be reliable, reproducible and precise. Method recovery was performed by correlating results obtained for analyte spiked samples at 4 (LLQC, LQC, MQC and HQC) concentrations with un-spiked standards.
Matrix effects
The blank plasma effect on the investigation of asciminib along with internal standard was evaluated in matrix effect. For this, the analyte free plasma of different batches was spiked with LQC and HQC level concentration of both asciminib and internal standard. Then the analytes were extracted and analysed. The area response of asciminib and internal standard was tabulated and the peak area response ratio of asciminib and internal standard was calculated. The % RSD of <15% was considered to be having acceptable matrix effect as per the guidelines.
Dilution integrity
This study was conducted to determine the influence of sample dilution on the precision and accuracy of asciminib in the proposed method. In this, the analytes were extracted from the plasma sample spiked with 5 times higher than HQC level. Then it was diluted to HQC and LQC levels and then analysed. The precision and accuracy was calculated in this study and results under ± 15% was considered as acceptable.
Stability experiments
The stability of asciminib in the proposed method was determined by conducting different stability studies like freeze-thaw, auto-sampler, dry extract, reinjection, short and long term. All stability studies utilized a set of standards prepared from fresh stock solutions of asciminib and internal standard in an appropriate analyte and analyte-free plasma matrix. The known concentration of sunitinib and internal standard was prepared for evaluating the stability. In all the performed stability studies, the acceptable limit was considered as the % accuracy and %RSD deviation of ± 15%.
In short term stability, 6 aliquots of HQC and LQC concentrations of asciminib and internal standard was preserved for 24 h and then analyzed. The long term stability was performed by storing the HQC and LQC concentrations of asciminib at -20°C ± 5°C and was analysed in every 3 days of analysis. Care should be taken while preparing the sample such that the sample volume must adequate for analysing 3 separate occasions. The % stability and the %RSD (Relative Standard Deviation) in each time interval were calculated and the long term stability period of asciminib in the developed method was established.
In freeze thaw stability, the HQC and LQC level solution of asciminib was kept in an air oven at 60°C ± 5°C for 24 h and thawed at room temperature. Then the sample was kept in a refrigerator -20°C ± 5°C for 24 h and then thawed at room temperature. This was considered as 1 freeze thaw cycle and the process was continued to 3 cycles. Then the sample was analyzed in the proposed method and results were utilized for evaluating the stability of asciminib.
The auto-sampler stability study was performed to evaluate the effect of infrequent delay in sample injection on stability of asciminib. In this, the sample at HQC and LQC level was incubated 24 in auto-sampler and then analysed. The dry reside of analytes after extraction was preserved 24 h at room temperature without reconstitution and then analysed immediately after reconstitution in dry extract stability. Simultaneously a fresh spiked analysis was performed and result obtained in each stability study was compared with fresh spiked analysis results and the % stability was calculated in each study.
Results and Discussion
To investigate the quantity of asciminib in plasma samples as well as to evaluate its stability one should adopt suitable method for its quantification. In recent times, rapid and very sensitive analytical technique like LCMS/MS was usually used for bio-analysis of drugs as well as its metabolytes. As the literature doesn’t show any analytical method for quantification of asciminib in biological samples, this study proposed to develop a simple and sensitive LCMS/MS method to quantify asciminib in biological samples like human plasma.
The mass operating conditions were tuned using spiked plasma solution containing 50 ng/mL of asciminib and 100 ng/mL of sunitinib internal standard. Intensity of both parent and fragment ion were observed to be significantly prominent in positive mode than negative mode.
The consistent product ion spectra with high intense fragment ions for both asciminib and internal standard were achieved by suitable altering compound parameters like CE, EP, CEP and DP. In addition to the compound parameters, the source dependent parameters such as nozzle voltage, sheath gas flow rate, sheath gas temperature, nebulizer pressure etc., were optimized to achieve satisfactory response of parent and fragment ions. An efficient and fast collision cell which was operated at suitable dwell to enable acquisition with enough data points that produce narrow peaks with nominal sensitivity lose and no cross-talk. Various optimizations were performed and a dwell time of 220 ms was noticed to be suitable with no cross-talk.
After optimizing mass operating parameters, the chromatographic parameters like column configuration, composition and flow of mobile phase were favourably optimized to achieve better quantification results. In the process of method development, methanol and acetonitrile were treated as best organic modifier proposed for LCMS analysis and hence were optimized with different combinations of pH buffers like aceate, phosphate and citrate. Based on the results achieved in each optimization study, the buffer solution containing ammonium formate was confirmed as suitable pH modifier and methanol was finalized as suitable organic solvent for resolving asciminib and internal standard. In the optimization of method development, different configurations of chromatographic columns such as C18 and C8 of different manufacturers was studied to achieve well resolved and retained peaks with short chromatographic run time and free from endogenous components with satisfactory response for both asciminib and internal standard.
The best chromatographic result was obtained using aqueous ammonium formate (5 mM) and methanol in 45 (v/v):55 (v/v) at pH 4.3 as a mobile phase under isocratic conditions. Alltima HP C18 (100 mm × 4.6 mm, 3 μm) column resolve the analytes with acceptable symmetry and acceptable peak response LLOQ concentration (2.5 ng/mL) level. The flow rate of mobile phase was optimized and best resolution of analytes with acceptable peak symmentry was achieved at 0.5 mL/min flow and a shortest 4 min run time to complete the analysis.
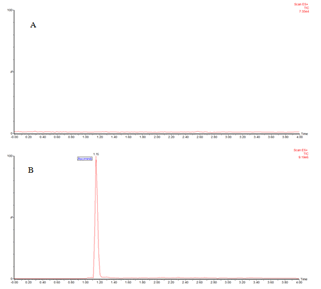
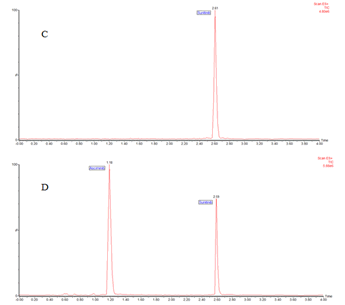
Selectivity and specificity of the optimized method was evaluated by analysing extracts obtained from blank human plasma, combined as well as individually spiked fixed concentrations of asciminib and sunitinib. As shown in Figure 2, there is no detection of direct interference of plasma traces at the retention time of the asciminib and internal standard. Similarly, (plasma spiked with asciminib) and (plasma spiked with internal standard) confirms that no direct interference to the MRM channel of the analyte. The system suitability chromatogram shown in Figure 3 in the developed method produces symmetric peak at elution time of 1.18 min for asciminib and 2.59 min for sunitinib and noticed to be produce satisfactory peak properties as per the guidelines (Figures 4 and 5).
Figure 2: Chromatogram identified in system suitability study A) LC chromatogram identified for plasma spiked with no analyte; B) spiked with asciminib
Figure 3: Chromatogram identified in system suitability study C) spiked with both standard asciminib; D) internal standard
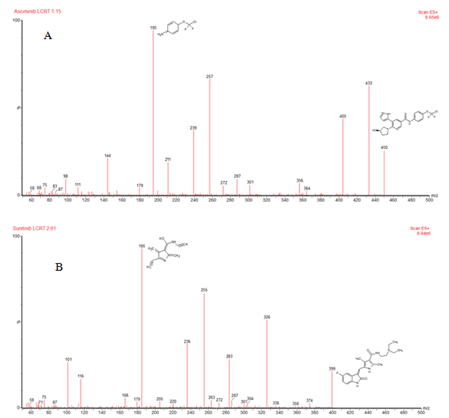
Figure 4: A) Mass spectra observed in the developed method showing parent and product ion [M+H]+ of asciminib; B) internal standard
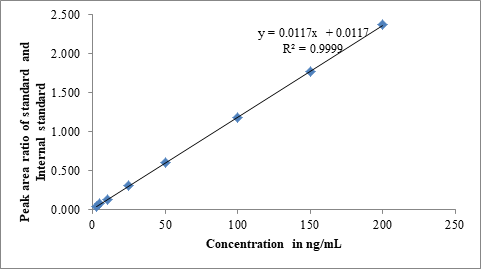
Figure 5: Linear calibration curve
In the optimized conditions, the Q1 full scan mass spectra shows major protonated [M+H]+ parent ions at m/z of 450 (m+1) and 399 (m+1) for asciminib and internal standard respectively. The most sensitive mass transition was noticed at m/z 195 (m+1) and 185 (m+1) for asciminib and internal standard respectively.
The reported method employed a convenient, inexpensive and rapid liquid-liquid extraction procedure for extracting analytes form plasma. In the process of extraction, different solvents like ethyl acetate, diethyl ether, tert–butyl methyl ether, dichloromethane and hexane were utilized as solvents for extracting asciminib and internal standard from the plasma. These solvents were tried in individually or combined with each other and in combination with buffers for extracting the analytes. This liquid-liquid extraction avoids the entry of non-volatile compounds on to the column and mass system by producing spectroscopically clean sample and also the cost of the experiment was very low. In the use of solvents other than tert–butyl methyl ether as extracting solvent produces poor recovery of both standard and internal standard. The clean extracts with high recovery were observed during the use of tert–butyl methyl ether as extracting solvent. The addition of additives such as formic acid, ammonium formate and ammonium acetate doesn’t influence the % recovery of analytes and hence were not used during the extraction process.
The calibration curve was plotted in the concentration level of 2.5 ng/mL to 200 ng/mL with y=0.0117x+0.0117 (R²=0.9999) as regression equation for asciminib. The linearity results presented in Table 1 and linear plot presented suggest that the proposed method gives elaborative and sensitive calibration which was adequately suitable for trace level analysis of asciminib.
Table 1: Linearity results
| S No | Concentration in ng/mL | Peak area of asciminib | Peak area of internal standard | Peak area ratio of asciminib and internal standard |
|---|---|---|---|---|
| 1 | 2.5 | 15285.9 | 415957.3 | 0.037 |
| 2 | 5 | 29854.7 | 416310.8 | 0.072 |
| 3 | 10 | 54157.6 | 414515.9 | 0.131 |
| 4 | 25 | 129247.1 | 416008.7 | 0.311 |
| 5 | 50 | 251573.6 | 415979.1 | 0.605 |
| 6 | 100 | 488180.4 | 415470.5 | 1.175 |
| 7 | 150 | 735821.3 | 416326.7 | 1.767 |
| 8 | 200 | 985147.8 | 415842.5 | 2.369 |
Table 2 presents the summarized results achieved in precision and accuracy study of asciminib in the study. The precision (%RSD) and accuracy values of asciminib was calculated and was noticed to be within the range of 98.13%–100.45% in intraday precision, 97.72%-100.75% in interday precision and 98.17%-100.44% in ruggedness study. The % RSD in each studied level was observed to be with in the acceptable level and results revealed acceptable precision and accuracy of the method.
Table 2: Precision and accuracy results of asciminib in the developed method
| S No | Quality control level | Concentration prepared (ng/mL) | Concentration found (ng/mL) | % accuracy | % Precision (RSD) |
|---|---|---|---|---|---|
| Intraday precision (n=6 at each level) | |||||
| 1 | LLOQ | 2.5 | 2.48 ± 0.033 | 99.09 ± 1.334 | 1.35 |
| 2 | LQC | 5 | 4.91 ± 0.038 | 98.13 ± 0.762 | 0.78 |
| 3 | MQC | 50 | 50.22 ± 0.791 | 100.45 ± 1.582 | 1.57 |
| 4 | HQC | 200 | 199.93 ± 0.666 | 99.97 ± 0.333 | 0.33 |
| Interday precision (n=6 at each level) | |||||
| 5 | LLOQ | 2.5 | 2.47 ± 0.033 | 98.65 ± 1.300 | 1.32 |
| 6 | LQC | 5 | 4.89 ± 0.047 | 97.72 ± 0.938 | 0.96 |
| 7 | MQC | 50 | 50.37 ± 0.809 | 100.74 ± 1.617 | 1.61 |
| 8 | HQC | 200 | 198.20 ± 0.740 | 99.10 ± 0.370 | 0.37 |
| Ruggedness (n = 6 at each level) | |||||
| 9 | LLOQ | 2.5 | 2.47 ± 0.034 | 98.84 ± 1.349 | 1.36 |
| 10 | LQC | 5 | 4.91 ± 0.026 | 98.17 ± 0.515 | 0.52 |
| 11 | MQC | 50 | 50.22 ± 0.791 | 100.44 ± 1.583 | 1.58 |
| 12 | HQC | 200 | 198.96 ± 0.702 | 99.48 ± 0.351 | 0.35 |
The extraction efficiency of asciminib and internal standard in the studied method was evaluated in recovery study by comparing aqueous calibration results. This experiment was conducted in HQC, MQC, LQC and LLOQ levels. The acceptable % recovery of 96.66 ± 0.302, 91.92 ± 0.592, 90.26 ± 0.387 and 86.21 ± 0.411 in HQC, MQC, LQC and LLOQ respectively was noticed for asciminib. Hence it can be confirms that the proposed extraction procedure was adequate for effective extraction of asciminib in plasma samples. Table 3 presents the recovery results of asciminib in this method.
Table 3: Recovery results of asciminib in the developed method
| S No | Recovery level | Concentration prepared (ng/mL) | Concentration recovered (ng/mL) | % Recovery | % RSD of recovery |
|---|---|---|---|---|---|
| 1 | HQC | 200 | 193.32 ± 0.605 | 96.66 ± 0.302 | 0.31 |
| 2 | MQC | 50 | 45.96 ± 0.296 | 91.92 ± 0.592 | 0.64 |
| 3 | LQC | 5 | 4.51 ± 0.019 | 90.26 ± 0.387 | 0.43 |
| 4 | LLOQ | 2.5 | 2.16 ± 0.010 | 86.21 ± 0.411 | 0.48 |
The effect of co-eluting matrix constituents on the extraction and analysis of asciminib in presence of internal standard was evaluated in matrix effect. Matrix effect of both asciminib and internal standard was evaluated in 3 levels in calibration curve i.e HQC, LQC and MQC. There is no enhancement or suppression results correspond to asciminib and internal standard was noticed suggest that no considerable matrix effect was noticed in the developed method. Hence the proposed extraction procedure and analytical method can effectively remove any probable interfering compounds from plasma matrix.
The dilution integrity study was conducted to determine the influence of sample dilution on precision and accuracy of asciminib and internal standard in the proposed method. Based on the peak area response archived in dilution integrity study, the % recovery and accuracy was calculated by comparing standard calibration curve. The % accuracy was calculated to be more than 96% for asciminib in the developed method suggest precise and accuracy of proposed method.
In the process of bioanalytical method development, adequate procedure must be used to preserve the stability of the sample because, instability of the drug produces unreliable data. Hence stability studies need to be performed to determine analyte stability in the developed conditions. A variety of stability experiments namely bench top, freeze– thaw, long term, auto-sampler and reinjection stability had been performed throughout validation.
In all the studied stability experiments, the % stability was calculated to be within ± 15% for asciminib in both HQC and LQC levels. The %RSD values in precision study of stability experimnets was calualted to be with in the acceptable limit of less than 15% (Table 4). The results achieved in stability study suggest that the method was stable over the entire validation range.
Table 4: Stability studies result of asciminib in the developed method
| S No | Stability test | QC level | Amount found (ng/mL) | % stability | % RSD |
|---|---|---|---|---|---|
| 1 | Bench top stability | HQC (200 ng/mL) | 199.99 ± 1.051 | 99.99 ± 0.526 | 0.53 |
| 2 | LQC (5 ng/mL) | 4.79 ± 0.137 | 95.84 ± 2.748 | 2.87 | |
| 3 | Freeze–thaw stability | HQC (200 ng/mL) | 199.55 ± 4.252 | 99.77 ± 2.126 | 2.13 |
| 4 | LQC (5 ng/mL) | 4.73 ± 0.206 | 94.62 ± 4.118 | 4.35 | |
| 5 | Long term stability | HQC (200 ng/mL) | 195.88 ± 7.500 | 97.94 ± 3.750 | 3.83 |
| 6 | LQC (5 ng/mL) | 4.89 ± 0.259 | 97.70 ± 5.179 | 5.3 | |
| 7 | Auto-sampler stability | HQC (200 ng/mL) | 194.84 ± 1.303 | 97.42 ± 0.651 | 0.67 |
| 8 | LQC (5 ng/mL) | 4.71 ± 0.084 | 94.29 ± 1.686 | 1.79 | |
| 9 | Reinjection stability | HQC (200 ng/mL) | 193.71 ± 0.621 | 96.86 ± 0.311 | 0.32 |
| 10 | LQC (5 ng/mL) | 4.86 ± 0.031 | 97.23 ± 0.617 | 0.63 |
Conclusion
A simple and convenient HPLC–MS/MS method was proposed and validated according to commonly acceptable US FDA guidelines for quantification of asciminib in biological samples like human plasma using similar class drug sunitinib as internal standard. The reproducible and consistent recovery of asciminib and internal standard was archived with an easy and convenient liquid-liquid extraction using methyl tertiary butyl ether as extracting solvent. The proposed method produces plasma recovered linear calibration curve in concentration level of 2.5 ng/ mL–200 ng/mL for asciminib. The method shows precision and accuracy results in HQC, MQC and LQC levels were within the permissible levels. The stability asciminib in the proposed method was evaluated by performing extensive stability studies such as bench top, freeze–thaw, long term, auto-sampler and reinjection stability. Acceptable results were produced in the developed method for asciminib in all the stability studies proved the stability of the method. Based on achieved results in all method validation studies, it can be confirmed that the method was sufficient for analysis and therapeutic monitoring of asciminib.
Acknowledgement
None.
Conflict Of Interest
Authors have no conflict of interest to declare.
References
- M.M. Moein, A.E. Beqqali, M.A. Rehim, Bioanalytical method development and validation: Critical concepts and strategies, J Chromatogr B Analyt Technol Biomed Life Sci, 1043(2017):3-11.
- G.I. Rahul, Z. Su, J. Huidi, F. Wei-Jie, Current developments of bioanalytical sample preparation techniques in pharmaceuticals, J Pharm Anal, 12(2022):517-529.
- G. Oskar, E.B. Maria, I. Gorka, B. Luis, I.M. Miren, et al. Bioanalytical chromatographic method validation according to current regulations, with a special focus on the non-well defined parameters limit of quantification, robustness and matrix effect, J Chromatogr A, 10(2014):233-7.
- M. Breccia, G. Colafigli, E. Scalzulli, M. Martelli, Asciminib: An investigational agent for the treatment of chronic myeloid leukemia, Expert Opin Investig Drugs, 30(2021):803-811.
- T.Y. David, S. Naranie, P.H. Timothy, Asciminib: A new therapeutic option in chronic-phase CML with treatment failure, Blood, 139(2022):3474-3479.
- US Department of Health and Human Services, FDA guidance for industry: Bioanalytical method validation. 2013.
Copyright: © 2023 Govindarao Yedlapalli, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.