Research Article - Journal of Drug and Alcohol Research ( 2023) Volume 0, Issue 0
Drug Discovery Analysis for Identification of Therapeutic Agents against Aspergillus Fumigates
Ajay Kumar* and Darakshan JabinAjay Kumar, Department of Biotechnology, Rama University, Kanpur, India, Email: ajaymtech@gmail.com
Received: 02-Jan-2023, Manuscript No. JDAR-23-88818; Editor assigned: 04-Jan-2023, Pre QC No. JDAR-23-88818 (PQ); Reviewed: 18-Jan-2023, QC No. JDAR-23-88818; Revised: 23-Jan-2023, Manuscript No. JDAR-23-88818 (R); Published: 30-Jan-2023, DOI: 10.4303/JDAR/236217
Abstract
Background: Traditional ayurvedic herbs contain many photochemical that are of therapeutic importance. Indian subcontinent is rich in therapeutic medicines based on plant compounds, and herbal medicines are the source for anti-fungal and anti-bacterial compounds. As we know, long term consumption of Aspergillus, Fusarium, and Penicillium mycotoxins has indeed been linked to renal and hepatic cancers, immune disorders, and many other pathophysiological disorders. Aspergillus fumigatus is a type of fungi that can cause a wide range of diseases in humans and animals, including allergic reactions, infections, and toxicity. It is one of the most common causative agents of opportunistic fungal infections, particularly in immunocompromised individuals. These infections can lead to severe respiratory distress, lung damage, and even death if left untreated.
Methodology: In current study we considered variety of herbal medicinal plants and there phytochemicals to reveal their interaction pattern with crucial enzymes of Aspergillus fumigates. In current study we aimed to conduct in-silico analysis for revealing the anti-fungal nature of phytochemicals Flavan-3-ol, Baicalein, Gallotannin 5, 8-Dihydroxyumbelliprenin, Ellagic acid, Lambertianin A, and Punicalins. This study also involves Lipinski drug analysis, and fundamental ADME analysis of phytochemicals that assisted us in developing core medicinal background of phytochemicals.
Results: Here we found phytochemicals (Flavan-3-ol, Baicalein, 5,8-Dihydroxyumbelliprenin (Asacoumarin A), and Ellagic acid) inhibit the crucial enzymes of fungus Aspergillus fumigates like Chitinase, Phytase and Thiolase. The Molecular docking studies reveal binding energy in the range of -8 kcal/mol to -13 kcal/mol, and also MD-simulations shows stable docked complexes as per the estimated RMSD values ranging from 0.1 angstrom to 6 angstrom during the time span of 40 nano-seconds.
Conclusion: In current study we explored phytochemicals named as Flavan- 3-ol, Baicalein 5, 8-Dihydroxyumbelliprenin (Asacoumarin A), and Ellagic acid by in-silico methods to identify their inhibitory action on three metabolic enzymes ChitinaseA, Phytase, and Thiolase of Aspergillus fumigates. It was found that these phytochemicals shows perfect docking in the binding pocket of these enzymes as per the binding energy scores and inhibitory coefficient values. Also, the interaction patterns show multiple hydrophobic interactions as well as hydrogen bonding between drug ligands and enzymatic receptor molecules. So we can conclude CADD method is fast and efficient way to develop possible treatment strategy against deadly pathogens, and in our study these phytochemicals hold therapeutic properties against Aspergillus fumigates.
Keywords
Aspergillus fumigates; Molecular docking; Herbal medicines; Phytochemicals; Simulation; ADME analysis
Abbreviations
(RMSD) Root Mean Square Deviation; (MD) Molecular Dynamics; (CADD) Computer-Aided Drug Design
Introduction
For centuries, herbs were used as a traditional remedy. Traditional ayurveda phytomedicines have long been recognized for their abundant resources that provide better treatment alternatives. Despite the availability of antifungal agents, treatment of Aspergillus fumigatus infections remains challenging due to the increasing incidence of drug resistance, side effects of current treatments, and limited efficacy. Therefore, there is a pressing need for the identification of new and effective therapeutic agents that can target Aspergillus fumigatus and mitigate the adverse effects of infection. Drug discovery analysis, a multi-disciplinary approach that combines computational, experimental, and chemical methods, has emerged as a powerful tool for the identification of new therapeutic agents against Aspergillus fumigates. This process involves screening a large number of chemical compounds, both naturally occurring and synthetic, for their ability to inhibit the growth and virulence of the fungus. In-silico models and high-throughput screening techniques are used to rapidly identify promising candidates, which are then subjected to in vitro and in vivo testing to confirm their efficacy and safety. The goal of drug discovery analysis for Aspergillus fumigatus is to identify new and effective therapeutic agents that can target specific pathways or enzymes essential for the survival and growth of the fungus. This research not only holds the potential to revolutionize the treatment of Aspergillus fumigatus infections, but also provide a platform for the discovery of new antifungal drugs against other fungal pathogens. In recent studies many plants were identified to hold anti-fungal therapeutic properties, few are listed below in Table 1 [1-5].
Table 1: List of photochemical and there plant sources that holds anti-fungal properties.
| S. No. | Phytochemicals | Plant Source | Literature Source |
|---|---|---|---|
| 1 | Flavan-3-ol | Syzygium cordatum | [1] |
| 2 | Baicalein | Scutellaria baicalensis | [2] |
| 3 | Gallotannin | Scutellaria baicalensis | [2] |
| 4 | 5,8-Dihydroxyumbelliprenin | Ferula foetida | [3] |
| 5 | Ellagic acid | Punica granatum | [4] |
| 6 | Lambertianin A | Rubus idaeus | [5] |
| 7 | Punicalins | Punica granatum | [4] |
Long term consumption of Aspergillus, Fusarium, and Penicillium mycotoxins has indeed been linked to renal and hepatic cancers, immune disorders, free radical generation, as well as other teratogenic, cancerous, and mutagenesis consequences. Aspergillus fumigatus has been the most common airborne fungal infection in industrialised nations, and it produces expansive aspergillosis in susceptible people, which is typically deadly [6,7]. Proanthocyanidins, or polymeric compounds of flavan-3-ol monomers, are a kind of polyphenolic compound that may aggregate to greater amounts in the leaves, fruits, seeds, and barks of most land plants and have been shown to help in preventing illnesses including lung and heart diseases associated to fungal infection [8].
Plant-based compounds were evaluated against aflatoxigenic Aspergillus spp. in a recent research, and they discovered that six natural compounds, including baicalein, nobiletin, meso-dihydroguaiaretic acid, pinoresinol, syringaresinol, and celastrol, have antifungal activity [9]. Baicalein (noncytotoxic) at very low concentrations greatly suppressed A. fumigatus growth, biofilm formation, and adhesion in vitro, according to a recent research. Baicalein decreased FK severity, lowered fungal burden, and suppressed neutrophil infiltration and activity in A. fumigatus keratitis animals. Baicalein decreased the expression of thymic stromal lymphopoietin (TSLP) and TSLP receptor (TSLPR) in vivo and in vitro, as well as suppressing the mRNA and protein levels of inflammatory factors IL-1, IL-6, and TNF-α [10]. In one of the most recent study in order to investigate the protective, antifungal, and antimicrobial effects of synthetic polyphenols that are structurally similar to herbs-derived phytochemicals, gallotannins derived from d-lyxose, d-ribose, l-rhamnose, d-mannose, and d-fructose were designed and synthesised. These compounds were antimicrobial against Gram-positive organisms such as Staphylococcus aureus and Enterococcus faecalis, but not against mycobacteria. In lower doses, they were effective inhibitors and disruptors of S. aureus biofilms, and they also interacted with the quorum-sensing system [11]. Gallotannins have also been found to have antifungal properties against Aspergillus fumigatus. Many other phytochemicals like 5, 8-Dihydroxyumbelliprenin (also known as Asacoumarin A), Ellagic acid, Lambertianin A, Punicalins were also found to be of antifungal nature in current studies [12,13].
In current study we aimed to conduct in-silico analysis for revealing the anti-fungal nature of these phytochemicals that are enlisted in Table 1. The recent advancement of molecular docking and simulation studies assisted in developing novel treatment strategies against deadly pathogen Aspergillus fumigates. Computer assisted drug discovery approaches holds structural analysis as well as interaction analysis of available drug molecules in databases like Pubchem, Maybridge, Chembrige and Zinc to renowned receptors and enzymes of fungal origin from protein databank (PDB) [14].
This study also involves Lipinski drug analysis, and fundamental ADME analysis of phytochemicals that will assist in developing core medicinal background of phytochemicals. Many recent studies support in-silico drug designing approaches against major pathogens like Dengue virus, SARS-CoV2, Candida aurius and HCMV [15-19]. Available target an enzyme of Aspergillus fumigates in RCSBPDB is enlisted in Table 2, these enzymes interactions with phytochemicals enlisted in Table 1 were conducted in this study. The flow chart of proposed analysis scheme was represented in Figure 1.
Table 2: Target enzymes of Aspergillus fumigate and their role.
| S.No. | Aspergillus fumigatus Enzymes | RCSB PDB ID | Functioning of enzymes | Literature Source |
|---|---|---|---|---|
| 1 | ChainB-ChitinaseA | 2XVN | Target chitin molecule of other fungal species and act as defensive enzyme | [26] |
| 2 | Phytase | 1SK9 | It is required for breakdown of phytic acid to release phosphorus, crucial for growth and development | [27] |
| 3 | Cytosolic Thiolase | 6ARG | It initiates condensation of two acetyl-CoA molecules to create acetoacetyl-CoA in the cytoplasm, required in the production of ergosterol. Necessary for growth and development | [28] |
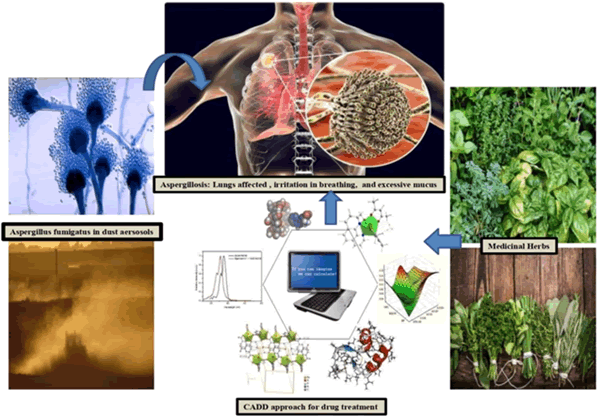
Figure 1: Medicinal herbs phytochemicals identification by using CADD approach against Aspergillus fumigates.
Methodology
Structure retrieval of phytochemicals
Phytochemicals structure was retrieved from Pubchem database (https://pubchem.ncbi.nlm.nih.gov/) with chemical ID enlisted in Table 3. SDF format structures downloaded and converted to PDB format. PDB format structures are required for docking analysis. SDF format 3D structures of phytochemicals were converted to 3D PDB structures by using Open babel software [20].
Table 3: Structure of phytochemicals retrieved from Pubchem database.
| S.No. | Phytochemicals | Pubchem Chemical ID | Structure |
|---|---|---|---|
| 1 | Flavan-3-ol | 3707243 |  |
| 2 | Baicalein | 5281605 |  |
| 3 | Gallotannin | 16133892 |  |
| 4 | 5,8-Dihydroxyumbelliprenin | 14313756 |  |
| 5 | Ellagic acid | 5281855 |  |
| 6 | Lambertianin A | 101632066 |  |
| 7 | Punicalins | 5388496 |  |
Structure retrieval of enzymes
Enzymes structure for Aspergillus fumigatus was retrieved from RCSB-PDB database (https://www.rcsb.org/). This database contains is ubiquitously recognized as a crucial and core data repository essential to basic and applied research in the life sciences, fundamental biology, and biomedicine, biotechnology, bioengineering, and energy communities. It is the world’s only worldwide compendium for three dimensional structure data, with over 170,000 experimentally obtained structures of proteins and their complexes with drugs and/or other moiety freely accessible without restrictions. Aspergillus fumigates enzymes ChainB-ChitinaseA, Phytase, and Cytosolic Thiolase structures were obtained in PDB format from this database.
ADME analysis of phytochemicals
SwissADME tool (http://www.swissadme.ch/) was used to conduct ADME analysis of phytochemicals [21]. Absorption, distribution, metabolism, and excretion (ADME) testing is becoming more common when for the number of potential chemicals. In this scenario, computer models are used, the SwissADME web tool, this provides free access to a large pool of fast yet reliable forecasting analytics for physicochemical characteristics, pharmacokinetic properties, and drug-likeness [21]. Druglikeness was analyzed on the basis of Lipinski rule of 5, which states that when there are more than 5 H-bond donors, 10 H-bond acceptors, the molecular weight is larger than 500, and the estimated Log Po/w is greater than 5, the Rule of 5 indicates that poor uptake or penetration is more likely. High lipophilicity of drug is indicated by Log Po/w value to be less than 5 which is good characteristic of drug [22].
Molecular docking analysis and 2D interaction pattern to explore H-bonds
Docking analysis was conducted by using Autodock vina software [23], which assisted in interacting phytochemicals screened after ADME analysis with enzymes of Aspergillus fumigatus. This analysis helped in revealing binding pocket and binding energy for the docked complexes. For this analysis ligand and enzyme preparation was conducted, then space was defined and interaction was revealed after docking. LigPlot+ ver.2.2.5 tool was used to explore hydrogen bond with in the docked complexes of drugs and enzymes [24].
Molecular dynamics and simulation analysis
To perform MD simulation analysis Gromacs software [25] was used, this tool assisted in revealing RMSD (Root Mean Square Deviation) plot for trajectory analysis at time span of 40 ns, also here we used OPLS-AA force field. All docked complexes were subjected to MD simulations to identify stability on the basis of RMSD and RMSF scores.
Results
Structure retrieval of phytochemicals
Phytochemicals structure was retrieved from Pubchem in SDF format and converted to PDB by using Open babel software, as per the literature sources presented in Table 1. The structures and Pubchem chemical IDs are presented in Table 3. Enzymes structures were retrieved from RCSBPDB database, and PDB IDs of three crucial enzymes was given in Table 2 [26-28].
ADME analysis of phytochemicals
SwissADME webserver was deployed to reveal drug properties and Lipinski violation for various phytochemicals under analysis. In Table 4 all the important parameters of drug likeness was provided as per the analysis. It was noted that out of 7 possible molecules from the literature only 4 molecules possess drug likeness against Aspergillus fumigatus, these four phytochemicals were Flavan-3-ol, Baicalein 5, 8-Dihydroxyumbelliprenin (Asacoumarin A), and Ellagic acid.
Table 4: ADME analysis of phytochemicals with Lipinski violations for druglikeness.
| S. No. | Phytochemicals | Formula | Molecular wt. | #Heavy atoms | #H-bond acceptors | #H-bond donors | Log Po/w | Lipinski violation | Inference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Flavan-3-ol | C15H14O2 | 226.27 | 17 | 2 | 1 | 2.52 | 0 | Accept |
| 2 | Baicalein | C15H10O5 | 270.24 | 20 | 5 | 3 | 2.43 | 0 | Accept |
| 3 | Gallotannin | C76H52O46 | 1701.2 | 122 | 46 | 25 | 6.2 | 4 | Reject |
| 4 | 5,8-Dihydroxyumbelliprenin | C24H30O5 | 398.49 | 29 | 5 | 2 | 4.43 | 0 | Accept |
| 5 | Ellagic acid | C14H6O8 | 302.19 | 22 | 8 | 4 | 0.79 | 0 | Accept |
| 6 | Lambertianin A | C82H54O52 | 1871.3 | 134 | 52 | 29 | 4.2 | 3 | Reject |
| 7 | Punicalins | C34H22O22 | 782.53 | 56 | 22 | 13 | 0.18 | 3 | Reject |
Molecular docking analysis
Molecular docking study was conducted between selected phytochemicals and target enzymes of Aspergillus fumigates. Autodock vina tool was used for conduction of docking analysis. In docking analysis it was found that 9 docked complexes were showing good binding energy and inhibition coefficient, as represented in Table 5. Using the formula Ki=exp (BE/RT), the inhibition coefficient (Ki) was calculated from the binding energy (G), where R is the universal gas constant (1.985 × 10-3 kcal mol-1 K-1) and T is the temperature (298.15 K) [29]. In Figure 2 all the 9 docked complexes were shown, to show there binding to the respective enzymes.
Table 5: Molecular docking analysis of phytochemicals and enzymes: Binding energies and Inhibition coefficient.
| Receptor | Ligand | Binding energy (BE) | Inhibition Coefficient (Ki) | Inference |
|---|---|---|---|---|
| ChainB-ChitinaseA | Flavan-3-ol | -9.8 | 6.381 | Accept |
| Baicalein | -10.2 | 3.245 | Accept | |
| 5,8-Dihydroxyumbelliprenin | -2.4 | 0.017 | Reject | |
| Ellagic acid | -2.1 | 0.028 | Reject | |
| Phytase | Flavan-3-ol | -11.3 | 5.054 | Accept |
| Baicalein | -10.8 | 1.176 | Accept | |
| 5,8-Dihydroxyumbelliprenin | -9.5 | 1.059 | Accept | |
| Ellagic acid | -8.6 | 4.852 | Accept | |
| Cytosolic Thiolase | Flavan-3-ol | -12.1 | 1.307 | Accept |
| Baicalein | -2.3 | 0.02 | Reject | |
| 5,8-Dihydroxyumbelliprenin (Asacoumarin A) | -12.6 | 5.61 | Accept | |
| Ellagic acid | -10.3 | 2.74 | Accept |
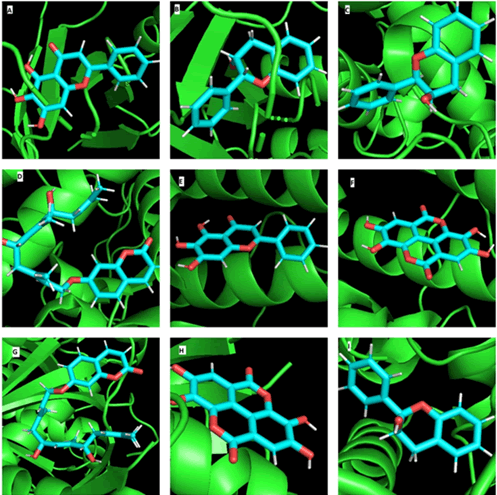
Figure 2: Molecular docking analysis: Docked complexes A) ChinaseA-Baicalein B) ChitinaseA-Flavan-3ol C) Phytase-Flavan-3ol D) Phytase-AsacoumarinA E) Phytase-Baicalein F) Phytase-Ellagic acid G) Thiolase-AsacoumarinA H) Thiolase-Ellagic acid I) Thiolase-Flavan-3ol.
2D Interaction analysis of docked complexes
LigPlot+ software was deployed for analyzing hydrophobic interactions, hydrogen bond interactions between ligand and receptor molecules in docked complex. ChitinaseA shows interaction with Baicalein and Flavan-3ol as represented in Figure 3, also it was noted that Baicalein makes hydrogen bond with Tyrosine residue at 181st position or Tyr (181) with bond length of 2.75 angstrom, and both Baicalein and Flavan-3ol makes many hydrophobic interactions with different amino residues of ChitinaseA justifying the higher value of binding energy and inhibition coefficient.
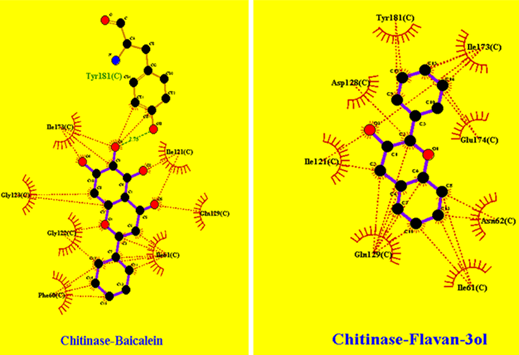
Figure 3: ChitinaseA interaction patterns: with Baicalein and Flavan-3ol phytochemicals.
In Figure 4 Phytase and AsacoumarinA interaction was marked, that clearly indicates Asacoumarin makes Hydrogen bonds of more than 3 angstrom with Asp (202), Gly (201), Thr (209), Lys (278), Tyr (28), and Asn (340) along with multiple hydrophobic interactions to Phytase enzyme; this results in to inhibition of active site of the Phytase enzyme. In Figure 5 Phytase and Baicalein interaction patterns were represented that clearly shows multiple hydrophobic interaction patterns. In Figure 6, Phytase interaction pattern with Flavan-3ol and Ellagic acid shows hydrogen bonding and multiple hydrophobic interactions.
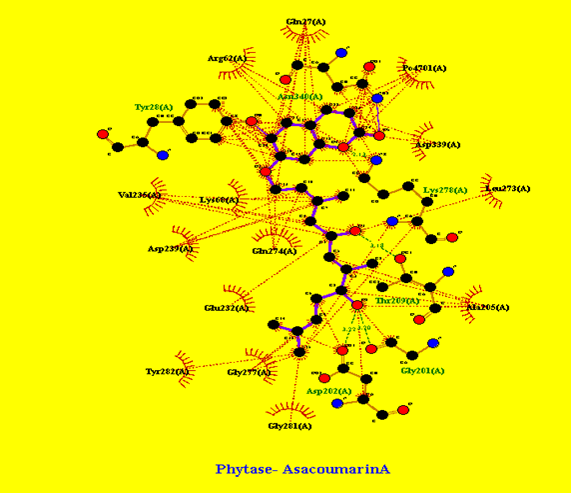
Figure 4: Phytase and AsacoumarinA interaction pattern: Hydrogen bonds of more than 3 angstrom with Asp (202), Gly (201), Thr (209), Lys (278), Tyr (28), and Asn (340) along with multiple hydrophobic interactions.
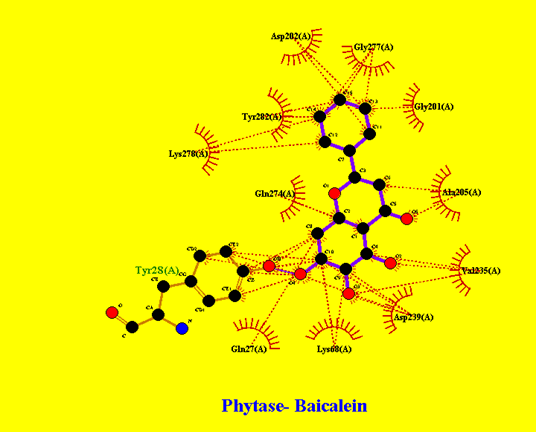
Figure 5: Phytase interaction pattern with Baicalein: Multiple hydrophobic interactions.
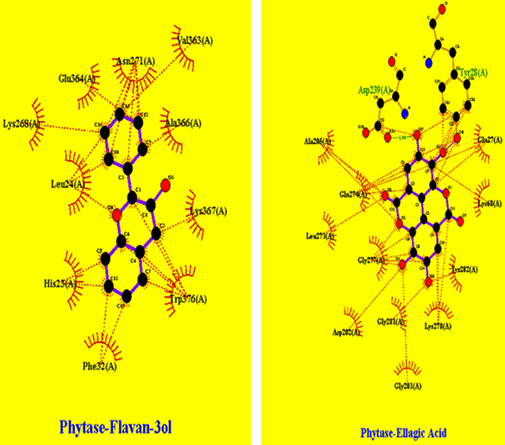
Figure 6: Phytase interaction pattern with Flavan-3ol and Ellagic acid: Hydrogen bond and multiple hydrophobic interactions.
In Figure 7 cytosolic Thiolase interactions with AsacoumarinA and Ellagic acid were shown, and in Figure 8, Thiolase interaction with flavan-3ol was shown were multiple hydrophobic interactions are indicated.
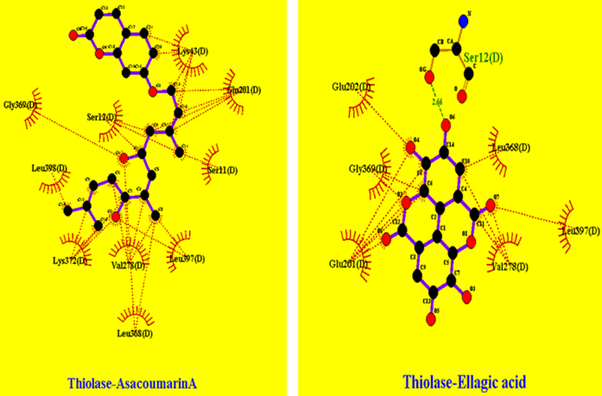
Figure 7: Thiolase interaction pattern with AsacoumarinA and Ellagic acid: showing hydrophobic interactions and hydrogen bonds (specifically Ellagic acid shows interaction with Serine residue at 12th position bond length of 2.66 angstrom).
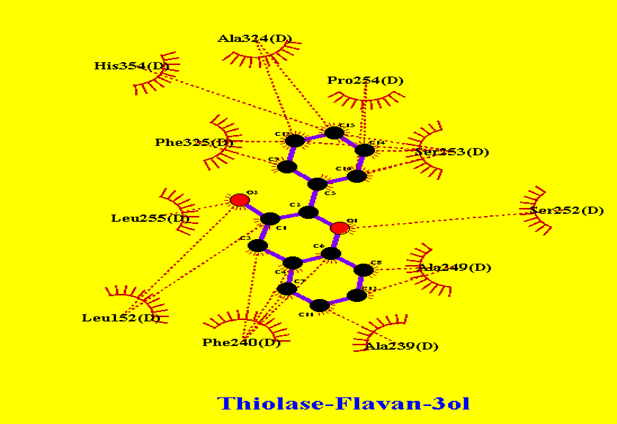
Figure 8: Thiolase interaction pattern with Flavan-3ol: showing multiple hydrophobic interactions.
MD-Simulations of docked complexes
RMSD (root mean square deviation) values were observed for all the 9 docked complexes, this indicates stable interaction of all docked complexes for time span of 40 ns. RMSD values were found to be under the 4 angstrom for considered docked complexes. In Figure 9 RMSD plot was shown.
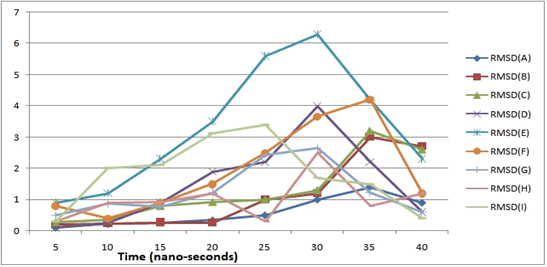
Figure 9: RMSD plot for finalized 9 docked complexes: Y-axis showing RMSD values in angstrom, and X-axis indicates time in nano-seconds (ns): (A) ChinaseA-Baicalein (B) ChitinaseA-Flavan-3ol (C) Phytase-Flavan-3ol (D) Phytase-AsacoumarinA (E) Phytase-Baicalein (F) Phytase-Ellagic acid (G) Thiolase-AsacoumarinA (H) Thiolase-Ellagic acid (I) Thiolase-Flavan-3ol.
Discussion
Aspergillus fumigates is a harmful pathogen as associated with multiple disorders like invasive aspergillosis and acute bronchopulmonary aspergillosis [30]. Currently fewer medications are available for the treatment of Aspergillus fumigates, and it’s a great challenge among scientific community to develop better treatment strategies against this harmfull fungal pathogen. In this study we deployed chemo-informatics approach for drug discovery. As in many previous studies it was observed computer-assisted drug designing approaches were used to develop regimens against recent pandemic viral pathogen SARSCoV2 [14,31]. In current study we found following docked complexes (A) ChinaseA-Baicalein, (B) ChitinaseA-Flavan- 3ol, (C) Phytase-Flavan-3ol, (D) Phytase-AsacoumarinA, (E) Phytase-Baicalein, (F) Phytase-Ellagic acid, (G) Thiolase-AsacoumarinA, (H) Thiolase-Ellagic acid, and (I) Thiolase-Flavan-3ol. These complexes show perfect interaction and formation of stable inhibitory complex of crucial metabolic enzymes of Aspergillus fumigates. Screening of ligands was done on the basis of available literature and Pubchem database, as conducted in many previous studies [32,33]. After the drug screening we initiated ADME analysis for further screening of drugs, on the basis of lipinski’s rule of drug-likeness. Then molecular docking and MD-simulation analysis was conducted. The Molecular docking studies reveal lesser binding energy in the range of -8 kcal/mol to -13 kcal/mol, and also MD-simulations shows stable docked complexes as per the RMSD values ranging from 0.1 angstrom to 6 angstrom during the time span of 40 nano-seconds. This study opens new avenues of research against the harmful pathogen Aspergillus fumigates. Wetlab validations will further strengthen the concept as a future scope of our research, as this method is not only economically efficient but also saves time of researchers from conventional cumbersome hit-and trial methods of drug designing.
Conclusion
In current study we explored phytochemicals named as Flavan- 3-ol, Baicalein 5, 8-Dihydroxyumbelliprenin (Asacoumarin A), and Ellagic acid by in-silico methods to identify their inhibitory action on three metabolic enzymes ChitinaseA, Phytase, and Thiolase of Aspergillus fumigates. It was found that these phytochemicals shows perfect docking in the binding pocket of these enzymes as per the binding energy scores and inhibitory coefficient values. Also, the interaction patterns show multiple hydrophobic interactions as well as hydrogen bonding between drug ligands and enzymatic receptor molecules. So we can conclude CADD method is fast and efficient way to develop possible treatment strategy against deadly pathogens, and in our study these phytochemicals hold therapeutic properties against Aspergillus fumigates.
Acknowledgement
All authors thanks to Rama University for providing best computational facility for conducting this research work
Conflict of Interest
All author’s state no conflict of interest.
Author's Contribution
AK, DJ conducted the research work and wrote manuscript
References
- R.K. Chalannavar, H. Baijnath, B. Odhav, Chemical constituents of the essential oil from Syzygium cordatum (Myrtaceae), Afr J Biotechnol, 10(2011):2741-2745.
- Q. Zhao, X.Y. Chen, C. Martin, Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants, Sci bull, 61(2016):1391-1398.
- S.M. Nabavi, M.A. Ebrahimzadeh, S.F. Nabavi, B. Eslami, A.A. Dehpour, Antioxidant and antihaemolytic activities of Ferula foetida regel (Umbelliferae), Eur Rev Med Pharmacol Sci, 15(2011):157-164.
[Google Scholar] [PubMed]
- J. Jurenka, Therapeutic applications of pomegranate (Punica granatum L.): A review, Altern Med Rev, 13(2008):128-44.
[Google Scholar] [PubMed]
- I.F. Ghalayini, M.A. Al-Ghazo, M.N. Harfeil, Prophylaxis and therapeutic effects of raspberry (Rubus idaeus) on renal stone formation in Balb/c mice, Int Braz J Urol, 37(2011):259-267.
[Crossref] [Google Scholar] [PubMed]
- J.P. Latgé, The pathobiology of Aspergillus fumigatus, Trends Microbiol, 9(2001):382-389.
[Crossref] [Google Scholar] [PubMed]
- Y. Ke, B. Ding, M. Zhang, T. Dong, Y. Fu, et al. Study on inhibitory activity and mechanism of chitosan oligosaccharides on Aspergillus Flavus and Aspergillus Fumigatus, Carbohydr Polym, 275(2022):118673.
[Crossref] [Google Scholar] [PubMed]
- J.H. Jun, N. Lu, M. Docampo-Palacios, X. Wang, R.A. Dixon, Dual activity of anthocyanidin reductase supports the dominant plant proanthocyanidin extension unit pathway, Sci Adv, 7(2021):4682.
[Crossref] [Google Scholar] [PubMed]
- F. Tian, S.Y. Lee, S.Y. Woo, H.Y. Choi, S.B. Park, et al. Effect of plant-based compounds on the antifungal and antiaflatoxigenic efficiency of strobilurins against Aspergillus flavus, J Hazard Mater, 415(2021):125663.
[Crossref] [Google Scholar] [PubMed]
- Y. Zhu, X. Peng, Y. Zhang, J. Lin, G. Zhao, Baicalein Protects against aspergillus fumigatus keratitis by reducing fungal load and inhibiting TSLP-induced inflammatory response, Invest Ophthalmol Vis Sci, 62(2021):26-26.
[Crossref] [Google Scholar] [PubMed]
- Z. Hricovíniová, S. Mascaretti, J. Hricovíniová, A. Čížek, J. Jampílek, New unnatural gallotannins: A way toward green antioxidants, antimicrobials and antibiofilm agents, Antioxidants, 10(2021):1288.
[Crossref] [Google Scholar] [PubMed]
- C.A. Monteiro, J.R.A. dos Santos, Phytochemicals and their antifungal potential against pathogenic yeasts, Phytochemicals in human health, 2019.
- M.S.A. Aboody, S. Mickymaray, Anti-fungal efficacy and mechanisms of flavonoids, Antibiotics, 9(2020):45.
[Crossref] [Google Scholar] [PubMed]
- A. Joshi, G. Sunil Krishnan, V. Kaushik, Molecular docking and simulation investigation: Effect of beta-sesquiphellandrene with ionic integration on SARS-CoV2 and SFTS viruses, J Genet Eng Biotechnol, 18(2020):1-8.
[Crossref] [Google Scholar] [PubMed]
- S. Krishnan, A. Joshi, V. Kaushik, T cell epitope designing for dengue peptide vaccine using docking and molecular simulation studies, Mol Simul, 46(2020):787-795.
- S. Krishnan, A. Joshi, N. Akhtar, V. Kaushik, Immunoinformatics designed T cell multi epitope dengue peptide vaccine derived from non structural proteome, Microb Pathog, 150(2021):104728.
[Crossref] [Google Scholar] [PubMed]
- N. Akhtar, A. Joshi, B. Singh, V. Kaushik, Immuno-informatics quest against COVID-19/SARS-COV-2: Determining putative T-cell epitopes for vaccine prediction, Infect Disord Drug Targets, 21(2021a):541-552.
[Crossref] [Google Scholar] [PubMed]
- N. Akhtar, A. Joshi, V. Kaushik, M. Kumar, M.A.U. Mannan, In-silico design of a multivalent epitope-based vaccine against Candida auris. Microb Pathog, 155(2021b):104879.
[Crossref] [Google Scholar] [PubMed]
- N. Akhtar, A. Joshi, J. Singh, V. Kaushik, Design of a novel and potent multivalent epitope based human cytomegalovirus peptide vaccine: An immunoinformatics approach, J Mol Liq, 335(2021c):116586.
- N.M. O'Boyle, M. Banck, C.A. James, C. Morley, T. Vandermeersch, et al. Open Babel: An open chemical toolbox, J Cheminform, 3(2011):1-14.
[Crossref] [Google Scholar] [PubMed]
- A. Daina, O. Michielin, V. Zoete, SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Sci Rep, 7(1):1-13.
[Crossref] [Google Scholar] [PubMed]
- C.A. Lipinski, F. Lombardo, B.W. Dominy, P. J. Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv Drug Deliv Rev, 23(1997):3-25.
[Crossref] [Google Scholar] [PubMed]
- O. Trott, A.J. Olson, AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J Comput Chem, 31(2010), 455-461.
[Crossref] [Google Scholar] [PubMed]
- R.A. Laskowski, M.B. Swindells, LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery, J Chem Inf Model, 51(2011):2778-86.
[Crossref] [Google Scholar] [PubMed]
- D. Van Der Spoel, E. Lindahl, B. Hess, G. Groenhof, A.E. Mark, et al. GROMACS: Fast, flexible, and free, J Comput Chem, 26(2005):1701-1718.
- L. Alcazar-Fuoli, C. Clavaud, C. Lamarre, V. Aimanianda, V. Seidl-Seiboth, et al. Functional analysis of the fungal/plant class chitinase family in Aspergillus fumigatus, Fungal Genet Biol, 48(2011):418-429.
[Google Scholar] [Crossref] [PubMed]
- D.M. Sanni, O.T. Lawal, V.N. Enujiugha, Purification and characterization of phytase from Aspergillus fumigatus Isolated from African giant snail (Achatina fulica), Biocatal Agric Biotech, 17(2019):225-232.
- A.C. Marshall, C.S. Bond, J.B. Bruning, Structure of Aspergillus fumigatus cytosolic thiolase: Trapped tetrahedral reaction intermediates and activation by monovalent cations, ACS Catal, 8(2018):1973-1989.
- C.L.D. Ortiz, G.C. Completo, R.C. Nacario, R.B. Nellas, Potential inhibitors of galactofuranosyltransferase 2 (GlfT2): Molecular docking, 3D-QSAR, and in silico ADMETox Studies, Scient Rep, 9(2019):1-28.
[Crossref] [Google Scholar] [PubMed]
- R. Thakur, R. Anand, S. Tiwari, A.P. Singh, B.N. Tiwary, et al. Cytokines induce effector T-helper cells during invasive aspergillosis: What we have learned about T-helper cells?, Front microbiol, 6(2015):429.
[Crossref] [Google Scholar] [PubMed]
- A. Simonis, S.J. Theobald, G. Fätkenheuer, J. Rybniker, J.J. Malin, A comparative analysis of remdesivir and other repurposed antivirals against SARS‐CoV‐2, EMBO Mol Med, 13(2021), e13105.
[Crossref] [Google Scholar] [PubMed]
- M. Butkiewicz, E.W. Lowe, R. Mueller, J.L. Mendenhall, P.L. Teixeira, C.D. Weaver, Benchmarking ligand-based virtual High-Throughput Screening with the PubChem database, Molecules, 18(2013):735-756.
[Crossref] [Google Scholar] [PubMed]
- H. Charoute, Z. Elkarhat, L. Elkhattabi, E. Fahime, N. Oukkache, et al. Computational screening of potential drugs against COVID-19 disease: The Neuropilin-1 receptor as molecular target, Virusdisease, 33(2022):1-9.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2023 Ajay kumar, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.