Research Article: Journal of Drug and Alcohol Research (2020) Volume 9, Issue 1
2Sarojini Naidu Vanita Pharmacy Maha Vidyalaya, India
Prakash Katakam, Department of Pharmaceutical Sciences, Acharya Nagarjuna University, India, Email: pkatakam9@gmail.com
Published: 30-Jan-2020
Abstract
Objective of the study: The aim of the present study is to prepare and evaluate biodegradable in situ gels of simvastatin for treatment of periodontal diseases. In the present research work is focused to formulate periodontal injectable in situ gels containing bone regenerating agent simvastatin, using biodegradable polymer.
Methodology: Appropriate amounts of polymer and solvent were weighed into 5 ml glass vials with proper airtight polypropylene caps. After initial mixing of the contents, vials were placed aside with occasional shaking overnight at room temperature to completely dissolve the polymer. Weigh accurately finely powdered drugs and add to above solution, close the lid and shake well and keep aside with occasional shaking and store in refrigerator at 8°C. The resulting solutions can be directly injected into subgingival pockets. The studies are further done to evaluate the prepared in situ gels for various pre-formulation studies, physicochemical characterization, drug content, in vitro drug release studies and stability studies.
Conclusion: Periodontal diseases are the conditions that affect the supporting structure of teeth leading to the formation of pocket due to which tooth loss occurs, for which site specific injectable drug delivery systems are gaining importance.
Based on physicochemical characteristics, the in situ gel formulation of simvastatin (F3) was optimized. It was concluded that in situ gel delivery system is a novel approach that can be developed for the treatment of Periodontitis. The present research showed that the optimized in situ gel formulations are more promising for successful delivery of simvastatin and to treat bone regeneration.
Introduction
Periodontitis can be classified based on disease activity (chronic or aggressive), etiology (specific bacterial, fungal or viral infection), by response to treatment (responsive or refractory), by site (localized or generalized). Chronic Periodontitis, formerly known as “adult periodontitis” or “chronic adult periodontitis” is the most prevalent form of periodontitis. It is generally considered to be a slowly progressing disease. However, in the presence of systemic or environmental factors that may modify the host response to plaque accumulation, such as diabetes, smoking or stress, disease progression may become more aggressive as shown in Figure 1 [1].

Figure 1: Standard calibration graph of SVS.
Methods
Gels
A gel is a solid or semisolid system of at least two constitutes, consisting of condense mass enclosing and interpenetrated by a liquid. When the coherent liquid is matrix and is rich in liquid, the product is often called a jelly and when the liquid is removed leaving only the framework, the gel is known as xero gels. In a typical polar gel, a natural or synthetic polymer builds a three dimensional matrix throughout a hydrophilic liquid [2].
In situ gel: The in situ gelling systems consist of polymer that exhibit sol-to-gel phase transitions due to change in specific physicochemical parameters in the environment [3].
Advantages of in situ system
1. These systems reduce toxic effects on the healthy tissue and reach sites that are conventionally inaccessible due to the presence of various barriers.
2. Increase the half-life of drugs, preventing their rapid degradation and reduce the rate of elimination, thus maintaining drug concentration within the therapeutic window.
3. Reduces the amount of drug required to achieve therapeutic efficacy.
4. Cut down the number of repeated invasive dosage required for certain conditions and thus help to improve patient’s compliance and offers better acceptability to patients.
Approaches of in situ gel drug delivery: There are certain broadly defined mechanisms used for triggering the in situ gel formation of biomaterials [4]:
a) Physiological stimuli (e.g., temperature and pH)
b) Physical mechanism changes in biomaterials (e.g., swelling and solvent exchange Diffusion)
c) Chemical reactions (e.g., ionic, enzymatic and photo initiated polymerization)
Materials and Methods
Materials
Simvastatin (SVS): A Gift Sample from MSN Labs, Hyderabad, Poly Capro Lactone(PCL)-Sigma aldrich, Hyderabad, Tetrahydrofuran-Sigma aldrich, Hyderabad.
Methods
Pre-formulation studies: Selection of analytical wavelength of simvastatin: 100 mg of Simvastatin was accurately weighed and dissolved in 100 ml of ethanol and the resulting solution is considered as stock solution. The stock solution was allowed to stand for 30 min and then filtered. From this filtered solution, 1 ml of solution was taken and diluted with pH 6.8 phosphate buffer up to 100 ml. The resulting solution was scanned between the wavelengths of 200 to 400 nm, the absorption maximum was found to be 238 nm and this wavelength was used for further studies [5].
Construction of calibration curve of simvastatin: 100 mg of Simvastatin was accurately weighed and transferred into 100 ml volumetric flask and dissolved in small quantity of ethanol. The volume was made up with pH 6.8 phosphate buffer and was considered as Stock solution (SS-I) (1000 μg/ml) [5].
From SS I, 10 ml was taken and diluted up to 100 mL with pH 6.8 phosphate buffer to get Stock II (SS-II) (100 μg/ml). From Stock II, 2, 4, 6 and 8 ml were taken and diluted up to 10 ml with pH 6.8 phosphate buffers to get 2, 4, 6, and 8 μg/ mL concentrated solutions respectively. The absorbance of resulting solutions is measured at 238 nm using Phosphate buffer as blank and a graph was plotted for concentration Vs absorbance to get calibration curve.
Compatibility studies
Compatibility of drug and polymers which are used to prepare in situ gels was established by infrared absorption spectra and DSC analysis [6].
FTIR spectral analysis: The FTIR spectra (400 to 4000 cm-1 and resolution of 4 cm-1) of the pure drug and polymers were measured by preparing dispersion in dry KBr using FTIR 8400S (Shimadzu Analytical India Pvt. Ltd., Mumbai, India). The transmission minima (absorption maxima) in the spectra obtained with these polymers were compared. The presence of additional peaks corresponding to the functional groups was noted.
Differential Scanning Calorimetry (DSC): DSC thermo grams were recorded for pure drug and the prepared formulation using a differential scanning calorimeter (Netzsch Technologies, Germany). Accurately weighed samples were placed on aluminium plates, sealed with aluminium lids, and heated at a constant rate of 5°C/min over a temperature range of 0°C-300°C.
Formulation of simvastatin in situ gel preparation: Formulation of Simvastatin in situ gels were designed based on the formulae given in the Table 1. The required quantity of polymer, PCL was dissolved in the specific quantity of tetrahydrofuran. After getting a clear solution, simvastatin was added and mixed using a magnetic stirrer for 20 min and then used for further studies. The formulations were prepared by keeping the drug concentration constant and varying the polymer solvent concentration.
Table 1: Formulation chart for Simvastatin in situ gel preparation.
| Formulations | SVS (mg) | PCL (mg) | Tetrahydrofuran (mg) |
|---|---|---|---|
| F1 | 10 | 20 | 180 |
| F2 | 10 | 50 | 150 |
| F3 | 10 | 80 | 120 |
| F4 | 20 | 20 | 180 |
| F5 | 20 | 50 | 150 |
| F6 | 20 | 80 | 120 |
Variables
IN VITRO Evaluation studies of prepared In situ gels [7].
Sol-gel transition temperature and gelling time: Sol-gel transition temperature may be defined as that temperature at which the phase transition from sol meniscus to gel meniscus is occurred. Gel formation is indicated by a lack of movement of meniscus on tilting the tube. Gelling time is the time for first detection of gelation.
The sol-to-gel phase transition temperature (gelation temperature) measured for all the prepared formulations. An aliquot of 2 ml refrigerated tested formulation was transferred to a test tube and sealed with a parafilm. The tube was maintained in a thermostatically controlled water bath at 4°C. The temperature of the water bath was increased gradually in increment of 3°C in the beginning of the experiment and then 1°C increment in the region of sol-gel transition temperature, the tested formulation was left to equilibrate for 10 min at each new setting. The gelation is considered to be occurred when the meniscus of the formula would no longer move upon tilting through angle 90°C.
Determination of pH: The pH of the gel was determined using a calibrated pH meter (manufactured by datla instruments, Hyderabad model number DI-45P). The readings were taken for average of 3 samples.
Determination of drug content
Drug content uniformity in the drug delivery system is an important aspect that determines the performance of the system in vivo conditions. If the drug is not distributed uniformly throughout the formulation, it could either lead to availability of sub therapeutic dose or toxic dose. Drug content uniformity was also performed to ensure minimum batch to batch variations. The prepared formulations were analyzed for the drug content by taking 1 ml of the in situ gel in 50 ml volumetric flask, 3 ml of pH 6.8 phosphate buffer was added and shaken to dissolve the drug, the volume was made up to the mark by pH 6.8 phosphate buffer and the solution was left overnight. The drug content was determined by measuring the absorbance in UV-Visible spectrophotometer.
Determination of viscosity
The viscosity studies of all the formulation were measured by using Brookfield digital viscometer (Brookfield DV II+, USA) with spindle number 21 at 250 rpm. Viscosity was measured at 4°C ± 1 and at 37 ± 1°C [8].
In vitro drug release studies
A modified procedure of was adopted for in vitro drug release studies of prepared formulations as reported by Katakam. For each formulation, using 1 ml disposal syringe, the preparation was drawn using #22 gauge needle and transferred into 1 ml simulated gingival fluid (pH 6.8 phosphate buffer) which is present in 5 ml glass vial and closed with polypropylene cap. Drug release studies were conducted by withdrawing 0.1 ml of the samples from vials at specified time intervals and replacing with the same volume with fresh buffer solution, until complete drug gets released from the formulation. The sample solution was transferred into 10 ml volumetric flask and made up to the mark with pH 6.8 phosphate buffer. Then the solution was filtered with the filter paper placed in the funnel which is placed in the other volumetric flask. The filtered solution was filled into cuvette and the U.V studies were performed by using the pH 6.8 phosphate buffer as the blank solution. The absorbance was noted and calculated for amount of drug release.
Mechanism of drug release
The different mathematical models may be applied for describing the kinetics of the drug release process from in situ gels; the most suited model is selected depending upon the experimental results. The kinetics of drug release from formulations were determined by finding the best fit of the release data using zero-order, first-order, Korsmeyer-Peppas and higuchi plots [9-11].
The Korsmeyer-Peppas equation is as follows;
Mt/M∞=1-A (exp-kt) (1)
log (1-Mt/M∞)=log A-kt/2.303 (2)
Where, Mt/M∞ is the fractional amount of drug released and t is the time in hrs. In this study, the release constant, k and constant, A were calculated from the slopes and intercepts of the plot of In (1-Mt/M∞) versus time t respectively where, Mt is the amount of drug release at time t; M∞ is the amount of drug release after infinite time; k is a release rate constant incorporating structural and geometric characteristics of the tablet; and A is the diffusional exponent indicative of the mechanism of drug release. To find out the release exponent, the log value of percentage drug dissolved was plotted against log time for each batch according to the above equation. If A is equivalent to 0.5 indicates Fickian (case I) release; greater than 0.5 but less than 1 for non-Fickian (anomalous) release and A is greater than 1 indicates super case II type of release. Case II generally refers to the erosion of the polymeric chain, and anomalous transport (Non-Fickian) refers to a combination of both diffusion and erosion controlled drug release.
Stability studies
The prepared formulations were packed in a screw capped bottle and studies were carried out for 12 months by keeping at 5 ± 2°C and 60 ± 5% RH; 30 ± 2°C and 65 ± 5% RH and for 6 months for accelerated storage condition at 40 ± 2°C and 75 ± 5% RH. Samples were withdrawn on 0, 3, 6 and 12 months for long term storage condition and 0, 3 and 6 months for accelerated storage condition and checked for changes in physical appearance and drug content as per ICH Q1A (R2) guidelines. Graphs were plotted using Sigma plot 12.0 to determine the statistical significance. Shelf life of the optimized formulation was calculated by using ‘Stab for R’ software. Acceptance limit of 90% of label claim is considered for determining shelf life.
Results and Discussion
Scanning of pure drug simvastatin
The pure drug is scanned by UV-visible spectroscopy in between the range of 200 nm-400 nm and the highest peak was observed at 238 nm when scanned using pH 6.8 phosphate buffer.
Standard calibration curve of simvastatin in pH 6.8 phosphate buffers: Standard calibration curve of SVS in pH 6.8 phosphate buffer was plotted by taking drug concentration versus absorbance. The absorbance against the drug concentrations of 2, 4, 6, 8 and 10 μg/ml were observed and found linear. The R2 value of SVS was obtained as 0.987 which shows good linearity of the calibration curve. The calibration absorbance data obtained is shown in Table 2, and the calibration curve is depicted graphically in Figure 1 respectively.
Table 2: Detailed clinical effects following kratom exposure by dose and coexposure.
| S.No | Concentration (µg/mL) | Absorbance ± SD |
|---|---|---|
| 1 | 2 | 0.053 ± 0.01 |
| 2 | 4 | 0.085 ± 0.02 |
| 3 | 6 | 0.108 ± 0.02 |
| 4 | 8 | 0.143 ± 0.02 |
| 5 | 10 | 0.178 ± 0.03 |
FTIR studies of SVS, PCL and optimized formulation: The IR spectra of pure simvastatin and optimized formulations are shown in Figure 2. The FTIR spectra obtained indicated that no chemical interaction occurred between the drug, polymers and the excipients used in formulating the in situ gel. But, a slight shift in absorption peaks position was noticed which indicated that physical interaction might have occurred between drug and the polymer Table 3.
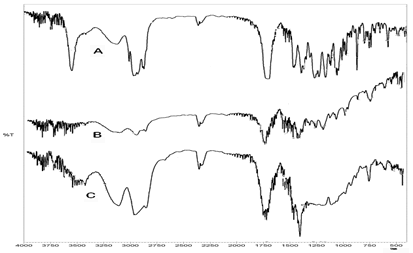
Figure 2: FTIR spectra of SVS (A), PCL (B) and optimised formulation (C).
Table 3: FT-IR spectral data obtained for simvastatin and formulation F3.
| Transition | IR Range (cm-1) | Absorption wave number | |
|---|---|---|---|
| SVS | Opt Formulation | ||
| C-0 stretching | 1400-1000 | 1103 | 1240 |
| -OH stretching | 3650-3300 | 3553 | 3560 |
| -OH bending | 1440-1400 | 1436 | 1440 |
| Para substituted Aromatic ring | 1600-1500 | 1595 | 1588 |
| Carboxylate | 1760-1665 | 1797 | 1770 |
| Amide | 1680-1630 | 1654 | 1660 |
| -C=CH stretching | 3100-3010 | 3069 | 3073 |
| Isopropyl bending | 1380-1360 | 1373 | 1368 |
| Aliphatic C-H Stretching | 2990-2850 | 2962 | 2968 |
From the Table 3, it can be noted that the characteristic IR peaks of SVS were seen at 1103(C-O stretching), 3553 (-OH stretching), 1436 (-OH bending), 1595 (para substituted aromatic ring), 1797 (carboxylate), 1654 (amide), 3069 (-C=CH stretching), 1373 (isopropyl bending) and 2962 (aliphatic C-H stretching) cm-1 respectively. The IR spectrum of the formulation F3 displayed the superimposition pattern of Simvastatin and polymer peaks with decreased peak intensity and little shifting of the peaks. The FTIR spectra of the pure drug as well as optimized formulation indicated that no chemical interaction occurred between Simvastatin and the excipients used. But, a slight shift in absorption peaks position was noticed. This result revealed that physical interaction occurred between drug and the polymer.
DSC study: DSC thermo grams of the pure drug SVS and its formulation were recorded to evaluate whether the drugs has undergone any degradation during the study period. From the DSC data obtained Figure 3, it was evident that the melting point of the drug, simvastatin has not changed after preparing the in situ gel formulation. Sharp endothermic peaks at 137°C and 139°C were obtained for pure drug and optimized formulation respectively. Hence, it may be inferred that there was no interaction between the selected drug and polymer. From DSC results, it may be concluded that the drug maintained its chemical identity in the formulation.
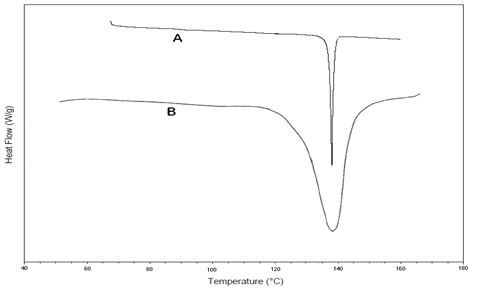
Figure 3: FT-IR spectral data obtained for simvastatin and formulation F3.
Physical properties of in situ gel: The prepared gels were evaluated for pH, viscosity and drug content and the obtained results are tabulated in Table 4.
Table 4: pH, viscosity and drug content for the prepared SVS in situ gels.
| Formulation Code | Surface pH | Viscosity (dynes/cm2) | Drug Content (% ± SD) |
|---|---|---|---|
| F1 | 5.9 ± 0.98 | 323 ± 1.34 | 100 ± 1.05 |
| F2 | 6.0 ± 1.04 | 359 ± 1.09 | 98 ± 1.76 |
| F3 | 6.1 ± 1.02 | 398 ± 0.90 | 99 ± 1.12 |
| F4 | 6.1 ± 0.89 | 387 ± 0.89 | 100 ± 2.10 |
| F5 | 6.2 ± 1.26 | 397 ± 1.84 | 101 ± 2.09 |
| F6 | 6.0 ± 0.33 | 396 ± 0.89 | 100 ± 1.20 |
From the Table 4, it is clear that the surface pH value for all formulations ranged between 5.9-6.1, which is the desired pH for in situ gels indicating that the formulations could be safely used and they do not cause any irritation in the oral cavity. From the above table, it is clear that as the concentration of PCL increased, the viscosity of the in situ gel increased. The maximum viscosity of 398 dynes/cm2 was seen for F3 formulation. The results of the Syringeability study indicated that all the gel formulations were syringeable i.e., they pass through 22 gauze needle and the gelation temperature was found to be 35°C.
In vitro release studies: The in vitro drug release data of Simvastatin from the prepared in situ gels is given in Table 5 and shown graphically in Figure 4.
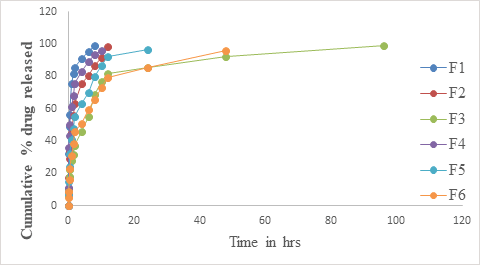
Figure 4: In vitro drug release of SVS in pH 6.8 phosphate buffer.
The In vitro drug release data of simvastatin from the prepared in situ gels is given in Table 5 and shown graphically in Figure 4. From the results, it is clear that F1, F2, F4, F5, F6 showed complete release of drug within 8, 12, 10, 24 and 48 hrs respectively, which means that they are not suitable for sustained drug release. However Formulation F3 showed sustained drug release until the study period of 96 hrs. Formulation F3 showed sustained drug release profile and the obtained data is fitted into release models to obtain the drug release kinetic mechanism.
Table 5: In vitro drug release data of formulations F1-F6.
| Time (Hours) | %drug release | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.083 | 10.46 | 8.69 | 5.49 | 11.09 | 7.09 | 5.12 |
| 0.166 | 32.53 | 17.48 | 10.91 | 35.98 | 14.98 | 8.62 |
| 0.333 | 48.76 | 29.47 | 18.17 | 43.47 | 24.47 | 16.23 |
| 0.5 | 56.63 | 33.34 | 22.26 | 50.36 | 32.36 | 23.34 |
| 1 | 75.88 | 45.23 | 28.22 | 61.13 | 41.3 | 31.35 |
| 1.5 | 81.62 | 55.54 | 31.47 | 68.27 | 47.7 | 38.38 |
| 2 | 85.5 | 63.27 | 37.57 | 75.47 | 55.47 | 45.7 |
| 4 | 90.99 | 75.43 | 45.91 | 83.14 | 62.98 | 51.18 |
| 6 | 95.5 | 80.53 | 55.02 | 89.41 | 70.14 | 59.38 |
| 8 | 99.4 | 86.63 | 68.53 | 93.74 | 79.95 | 65.92 |
| 10 | - | 91.95 | 76.89 | 96.08 | 86.54 | 72.98 |
| 12 | - | 98.76 | 81.54 | - | 92.55 | 79.35 |
| 24 | - | - | 85.63 | - | 96.6 | 85.35 |
| 48 | - | - | 92.07 | - | - | 96.32 |
| 96 | - | - | 99.12 | - | - | - |
Mathematical model fitting (Mechanism of drug release)
In vitro release studies data of prepared in situ gels was fitted into various mathematical models to determine the best fit model. The best fit model with the highest regression coefficients (R2) for the selected formulations is given in Table 6. The results indicated that, the best fit model was found to be Peppas model. From the Table, it was concluded that, formulation F3 with R2 value of 0.9961 is the optimized formulation for 96 hr study period.
Table 6: Model fitting data for the Simvastatin in situ gels.
| Release model | Formulation code | |
| F3 | ||
| Zero order | R2 | 0.9344 |
| First order | R2 | 0.9101 |
| Hixson Crowell | R2 | 0.8387 |
| Matrix | R2 | 0.8823 |
| Peppas | R2 | 0.9961 |
| n | 1.178 | |
| Best fit model | Peppas | |
Stability study of the gel: The optimized formulation, F3 was subjected to stability studies according to ICH guidelines by storing at 5 ± 2°C/60 ± 5% RH and 30 ± 2°C/65 ± 5% RH for 12 months and 40 ± 2°C/75 ± 5% RH for 6 months. The samples were analyzed and checked for changes in physical appearance and drug content at regular intervals. The obtained data is presented in Table 7. From the Table, it was clear that the formulations did not undergo any significant chemical change/interaction at 95% confidence interval during the study period.
Table 7: Stability study data of optimized formulation, F3.
| Stability condition | Sampling interval (months) | Physical appearance | % Drug content* |
|---|---|---|---|
| 5o ± 2°C/60 ± 5% RH | 0 | No change | 99.50 ± 0.50 |
| 3 | No change | 99.00 ± 0.22 | |
| 6 | No change | 98.81 ± 0.14 | |
| 12 | No change | 98.74 ± 0.42 | |
| 30o ± 2°C/65 ± 5% RH | 0 | No change | 99.50 ± 0.50 |
| 3 | No change | 98.92 ± 0.10 | |
| 6 | No change | 98.80 ± 0.14 | |
| 12 | No change | 98.62 ± 0.26 | |
| 40º ± 2°C/75 ± 5% RH | 0 | No change | 99.50 ± 0.50 |
| 3 | No change | 98.00 ± 0.60 | |
| 6 | No change | 97.20 ± 0.72 |
Summary and Conclusion
Periodontal diseases are the conditions that affect the supporting structure of teeth leading to the formation of pocket due to which tooth loss occurs, for which site specific injectable drug delivery systems are gaining importance.
The main experimental study was carried out to formulate in situ gels using different concentrations of drugs and polymer. The prepared in situ gels of various concentrations were evaluated for various properties such as viscosity, surface pH, drug content uniformity, gelation temperature, and in vitro drug release studies.
The results of the investigation undertaken are summarized as follows: Simvastatin selectively and competitively inhibit the hepatic enzyme HMG-CoA reductase. Statins have shown pleiotropic effects like anti-inflammation and bone stimulation. The results revealed that the formulation F3 was optimized by considering the various parameters such as:
1. The drug excipients compatibility studies showed that there was no interaction between the drug Simvastatin and the polymer PCL
2. The formulation changed from sol-gel at pH 6.8 and having a good viscosity and release.
3. The results revealed that the surface pH was within the range of gingival pH.
4. The gelation temperature was found to be 35°C.
5. The viscosity value of optimized formulation was found to be 389 dyne/cm2.
6. The drug content optimized formulation was found to be 99%.
7. Percentage drug release in formulation was 99.12% for about 96 hrs which was found to be most successful.
This study revealed that in situ gels formulation was simple, easy to administer, comfortable, with less side effects, has increased compliance and also enhanced the antimicrobial activity by releasing the drug in prolong manner. It was concluded that in situ gel delivery system is a novel approach that can be developed for the treatment of Periodontitis.
Conflict of Interest Disclosure
We declare that we have no conflicts of interest related to the authorship or publication of this manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request
References
- A. M. Cesar, Periodontal diseases and cancer, Lnct Oncol, 9 (2008), 510–512.
- S. B. Pedro, L. P. Ricardo, L. R. Rui, E. G. Manuela, Membranes for periodontal tissue regeneration, Ciencia and Technologia Dos Materials, 26(2014), 108-117.
- M. Madan, A. Bajaj, S. Lewis, N. Udupa, J. A. Baig, In situ forming polymeric drug delivery systems, Ind J Pharm Sci, 71 (2009), 242–251.
- I. A. Kanahaiya, M. Naveen, N. Ashish, K. G. Anil, In-Situ gel formation for ocular drug delivery system- An overview, Amrn J Bio Pharm Sci, 1 (2011), 01-07.
- S. Prasanthi, A. Rajendra Prasad, Y. Ganesh kumar, K. Shantha kumara, Development and validation of UV spectroscopy method for simvastatin in pH 6.8 phosphate buffer, Int J Pharm Anal Res, 4 (2015), 16-20.
- N. Shashank Nayak, S. Bharani, R. S. Thakur, Formulation and evaluation of pH triggered in situ ophthalmic gel of moxifloxacin hydrochloride, Int J Pharm Pharm Sci, 4 (2012), 452-459.
- V. D. Aarti, R. S. Mahendra, Thermoreversibal anesthetic gel for periodontal intrapocket delivery of mepivacaine hydrochloride, Schlr Rsrch Lib, 4 (2012), 889–896.
- D. W. Vijay, H. D. Ketaki, V. W. Kalpana, Formulation and Evaluation of in situ Gel Drug Delivery System of Sesbania grandiflora Flower Extract for the Treatment of Bacterial Conjunctivitis, J Pharm Sci and Res, 4 (2012), 1880-1884.
- M. Donbrow, Y. Samuelov, Zero order drug delivery from double-layered porous films: Release rate profiles from ethyl cellulose, hydroxypropyl cellulose and polyethylene glycol mixtures, J Pharm Pharmacol, 32 (1980), 463–70.
- N. A. Peppas, Analysis of fickian and non-fickian drug release from polymers, Pharm Acta Helv, 60 (1985), 110-116.
- R. W. Korsmeyer, R. Gurny, E. Doelker, P. Buri, N. A. Peppas, Mechanisms of solute release from porous hydrophilic polymers, Int J Pharm, 15 (1983), 25-35.

