Research Article - Journal of Drug and Alcohol Research ( 2023) Volume 12, Issue 4
GC-MS Analysis and Anti-cancer (Pancreatic Cancer) Activity of Syzygium Aternifolium and Smilax Chinensis: An In Vitro Study
Jiten Mishra*, Sudhir Kumar Sahoo, Susanta Kumar Panda and Nityashree MohapatraJiten Mishra, Department of Pharmacy, Biju Patnaik University of Technology, India, Email: jitenmishra000@gmail.com
Received: 03-May-2023, Manuscript No. JDAR-23-100074 ; Editor assigned: 05-May-2023, Pre QC No. JDAR-23-100074 (PQ); Reviewed: 19-May-2023, QC No. JDAR-23-100074 ; Revised: 24-May-2023, Manuscript No. JDAR-23-100074 (R); Published: 31-May-2023, DOI: 10.4303/JDAR/236238
Abstract
The objective of this present work is to identify the Phytochemical compounds by using GC-MS technique and its anticancer activity using PANC 1, BxPC-3, and HepG2 cell lines. In Syzygium alternifolium, 8 photochemical compounds and 10 compounds are from Smilax chinensis. These compounds are found to have significant anticancer activity against the selected cell lines and might be due to the presence of Hexadecanoic acid, Octadecenoic acid, and Cinnamic acid.
Keywords
GC-MS analysis; Cell lines; Anti-cancer activity
Introduction
Pancreatic cancer is the leading cause of cancer death worldwide and its global burden has more than doubled over the past 25 years. The regions with the highest incidence of pancreatic cancer include North America, Europe and Australia, and although much of this increase is due to the aging of the global population, there are key modifiable risk factors for pancreatic cancer such as cigarette smoking, obesity, diabetes and alcohol intake [1]. GLOBOCAN 2018 estimated that pancreatic cancer ranked as the 11th most common cancer worldwide in 2018 with 458,918 new cases and caused 432,242 deaths (4.5% of all cancer deaths) [2]. Despite advances in the knowledge of potential risk factors that cause pancreatic cancer and newly available tools for early diagnosis, its incidence is estimated to increase to include 355,317 new cases by 2040. Pancreatic cancer survival rates are influenced by several factors, such as age, sex, type of cancer, staging at diagnosis, tumor size, serum albumin level, treatments, differences and availability of health care systems, and other factors including overall health and lifestyle. Because pancreatic adenocarcinoma and other less common exocrine cancers are usually diagnosed at stage III or IV, it has a very poor prognosis compared to PanNET, and even in cases that cannot be treated surgically, the 5-year survival is 16% [3].
With conventional treatment, including surgical therapy and chemotherapy, the mortality rate is still high. Therefore, the possibility of using natural products for pancreatic cancer increases with plants that have mechanisms of anti-cancer effects such as apoptosis, anti-metastasis, anti- angiogenesis and anti-resistance [4]. Many plant isolates have been reported to have anticancer activity against pancreatic cell lines and preclinical models. Recent research on various forms of cancer, including pancreatic cancer, revealed that herbal medicines and natural products (HM/ NPs) isolated from plants could provide additional strategies for monotherapy or combination therapy, gingerol and shagaol (Red); thymoquinone (cumin), essential oil (holy basil); parthenolide (fever); Escin (horse chestnut) [5,6]. Therefore, this study is designed to identify the active phytochemicals present in Syzygium alternifolium and Smilax chinensis and later screen for in vitro anticancer activity using selected cell lines.
Materials and Methods
Analysis of plant extracts by GC-MS
GC-MS analysis was carried out by using Trace GC ultra- gas chromatograph connected to a Quantum XLS mass spectrometer (Thermo Scientific, FL, USA). GC was equipped with TG-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) consisting of a stationary phase of 5% phenyl and 95% methyl polysiloxane. Samples were dissolved in methanol and 1 μl of selected plant extract was injected into CT splitless mode at an injector temperature of 260°C. The flow rate of the carrier gas (helium) was 1 mL/min. The oven temperature was programmed from 110°C (isothermal for 2 minutes), with an increase of 10°C/min to 200°C, then 5°C/min to 280°C, ending with a 9 minute isothermal at 280°C. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 45 Da to 450 Da. The solvent delay was 0 minutes to 2 minutes, and the total GC/MS running time was 50 minutes. Interpretation on mass-spectrum GC-MS was conducted using the database of the National Institute of Standards and Technology (NIST). The spectrum of the unknown components was compared with the spectrum of known components stored in the NIST library. The name, molecular weight, and structure of the components of the test materials were identified.
MTT assay
The anticancer activity of the compounds was determined using an MTT assay [7,8]. 1 × 104 cells/well were seeded in 100 μl DMEM/MEM, supplemented with 10% FBS in each well of 96-well microculture plates, and incubated for 24 h at 37°C in a CO2 incubator. After incubation, all the prepared/synthesized compounds were added to the cells at 1 μg, 10 μg, 50 μg, and 100 μg concentrations for 48 h. After 48 h of drug treatment, 10 μl MTT (3-(4, 5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide) (5 mg/ mL) was added to each well and the plates were further incubated for 4 h. Then the supernatant from each well was carefully removed, formazan crystals were dissolved in 100 μl of DMSO and absorbance at 570 nm wavelength was recorded.
Results and Discussion
Phytochemicals are useful in the treatment of certain disorders by their individual, additive, or synergic actions to improve health. Phytochemicals are vital in the pharmaceutical industry for developing new drugs and preparing therapeutic agents. The development of new drugs starts with the identification of active principles from natural sources. The screening of plant extracts is a new approach to finding therapeutically active compounds in various plant species [9,10].
The medicinal power of traditional plant species lies in phytochemical components that cause definite pharmacological action in the human body. Based on their metabolism activity in the plant, phytochemicals components are generally can be mainly divided into two groups, which are primary which has mainly sugars, amino acids, chlorophyll, and proteins, and secondary constituents while secondary constituents consist of alkaloids, flavonoids, saponins, tannins, phenolic compounds and many more [11]. As proposed by WHO, the primary health care of most population of developing countries depend on traditional medicines and mostly natural plant products [12]. Phytochemical screening not only helps to reveal the constituents of the plant extracts and the ones that predominate over the others but also helps search for bioactive agents that can be used in the synthesis of useful drugs [13].
In the last few years, Gas Chromatography-Mass Spectrometry (GC-MS) has become firmly established as a key technological platform for secondary metabolite profiling in both plant and non-plant species [14]. The present study was conducted for GCMS analysis of Syzygium alternifolium and Smilax chinensis.
The possible compounds detected from the extract of Syzygium alternifolium via GC-MS analysis are presented in Table 1, with the respective chromatograms entered in Figure 1. The compounds were detected and identified by their fragmentation pattern and in conjunction with the NIST library. Analyses of GC-MS chromatogram showed the presence of 8 peaks which indicate 8 major phytochemical constituents with the highest concentrations. According the data revealed the presence of the following compounds: 3-Cyclopentene-1-acetaldehyde, 2,2,3-trimethyl; 1H-4-Azacycloprop[cd]indeoctahedron-4-methylthyl-; 1,2-Benzenedicarboxylic acid, butyl octyl ester; Benzene, (3-met3-methyl cyclopentyl-Hydroxy-beta-[carboxymethyl] cinnamic acid; Octanal, 2-phenyl methylene)-; Hexanedioic acid, bis(2-ethylhexyl) ester; 2-Cyclohexen-1-one, 4-(3-hydroxy-1-butenyl)-3,5,5-trimethyl-.
Table 1: The active principles in the Syzygium alternifolium extract with their Retention Time (RT), molecular formula, and Molecular Weight (MW)
| S.No | RT | Name of the compound | Molecular formula | Molecular weight | Structure |
|---|---|---|---|---|---|
| 1 | 13.99 | 3-Cyclopentene-1-acetaldehyde, 2,2,3-trimethyl | C10H16O | 152.2334 | 1 |
| 2 | 23.67 | 1H-4-Azacycloprop[cd]indene, octahydro-4-methyl- | C9H15N | 137.2 | 2 |
| 3 | 24.14 | 1,2-Benzenedicarboxylic acid, butyl octyl ester | C20H30O4 | 334.4498 | 3 |
| 4 | 24.52 | Benzene, (3-methylcyclopentyl)- | C12H16 | 160.2554 | 4 |
| 5 | 26.56 | p-Hydroxy-beta-[carboxymethyl]-cinnamic acid; | C12H14O4 | 222.24 | 5 |
| 6 | 30.08 | Octanal, 2-(phenyl methylene)- | C15H20O | 216.3187 | 6 |
| 7 | 42.13 | Hexanedioic acid, bis(2-ethylhexyl) ester | C22H42O4 | 370.5665 | 7 |
| 8 | 42.82 | 2-Cyclohexen-1-one, 4-(3-hydroxy-1-butenyl)-3,5,5trimethyl- | C13H20O2 | 208.297 | 8 |
The possible compounds detected from an extract of Smilax chinensis are presented in Figure 2. The compounds detected are identified by their fragmentation pattern and in conjunction with the NIST library. Analyses of GC-MS chromatogram showed the presence of 10 peaks which indicate 10 major phytochemical constituents with the highest concentrations. According the data revealed the presence of the following compounds-p-Hydroxy-beta-[ carboxymethyl]-cinnamic acid; Phenol, 2,5-bis(1,1-dimethyl ethyl)-; p-Hydroxy-beta-[carboxymethyl]-cinnamic acid; Octanal, 2-(phenyl methylene)-; Dibutyl phthalate; Hexadecanoic acid, ethyl ester; 9-Octadecenoic acid (Z)-, methyl ester; Ethyl Oleate; Hexanedioic acid, mono(2-ethylhexyl) ester; γ-Sitosterol (Table 2).
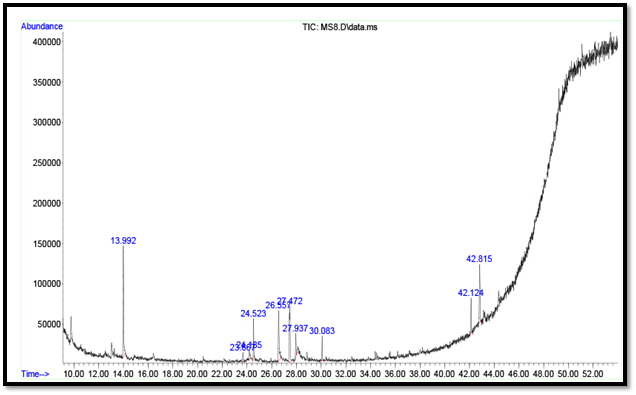
Figure 1: Identification of bioactive compounds in the extract of Syzygium alternifolium by GC-MS
Figure 2: Identification of bioactive compounds in the extract of Smilax chinensis by GC-MS
Table 2: The active principles in the Smilax chinensis extract with their Retention Time (RT), molecular formula, and Molecular Weight (MW)
| S. No | RT | Name of the compound | Molecular formula | Molecular weight | Structure |
|---|---|---|---|---|---|
| 1 | 24.49 | p-Hydroxy-beta-[carboxymethyl]-cinnamic acid; | C12H14O4 | 222.24 | 1 |
| 2 | 24.58 | Phenol, 2,5-bis(1,1-dimethyl ethyl)- | C14H22O | 206.3239 | 2 |
| 3 | 26.56 | p-Hydroxy-beta-[carboxymethyl]-cinnamic acid; | C12H14O4 | 222.24 | 3 |
| 4 | 30.09 | Octanal, 2-(phenyl methylene)- | C15H20O | 216.3187 | 4 |
| 5 | 34.37 | Dibutyl phthalate | C16H22O4 | 278.34 | 5 |
| 6 | 35.13 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284.4772 | 6 |
| 7 | 37.13 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | 296.4879 | 7 |
| 8 | 38.33 | Ethyl Oleate | C20H38O2 | 310.51 | 8 |
| 9 | 42.13 | Hexanedioic acid, mono(2-ethylhexyl)ester | C22H42O4 | 370.5665 | 9 |
| 10 | 47.72 | ?-Sitosterol | C29H50O | 414.7067 | 10 |
The Hexadecanoic acid has antioxidant, 5-alpha-reductase inhibitor, anti-fibrinolytic, hemolytic, antimicrobial activity, hypocholesterolemic nematicide, pesticide, antiandrogenic flavor, and hemolytic properties [10]. Octadecenoic acid has antioxidant activity and is in the treatment of stroke, acute neurologic disorder, respiratory failure, and anemia treatment [15]. Studies have reported that cinnamic acid exhibit antioxidant, antimicrobial, anticancer, neuroprotective, anti-inflammatory, and anti-diabetic properties [16,17]. Cinnamic acid terminates radical chain reactions by donating electrons that react with radicals forming stable products [18]. The Octanal is an aromatic aldehyde, with antioxidant and antimicrobial activities. Octanal shows cytotoxicity against Hela cells [19]. Both the selected plant materials were found to have potent phytochemicals reported to have many pharmacological properties.
Pancreatic Adenocarcinoma (PA) is an aggressive disease that develops in a relatively symptom-free manner and is usually advanced at the time of diagnosis. As is common in epithelial tumors, carcinogenesis develops through the accumulation of mutations and genetic lesions leading to the activation of oncogenes and the inactivation of tumor suppressor genes. Since multiple combinations of mutations can lead to the development of PA disease sub-classes may present different survival strategies requiring multiple targeted intervention strategies [20]. A thorough understanding of the specific cellular and molecular mechanisms of PA development and progression is required to identify early detection strategies, preventative measures, and effective interventions. Approximately 10% of pancreatic cancer patients have a family history of cancer; individuals with mutations in KRAS, TP53, GNAS, SMAD4, CDKN2A, RNF43, ARID1A, and KMT2C may be at higher risk. The CFPAC-1, BxPC-3, AsPC-1, PANC-1, and MIA PaCa-2 cell lines were used to screen the anticancer properties of drug conjugates to treat pancreatic cancer [21]. Liver metastasis is a common feature of pancreatic adenocarcinomas. More than 50% of patients with pancreatic cancer have liver metastases at the time of diagnosis which is associated with a poor prognosis. For patients with resectable disease, surgery is the treatment of choice, and it has been moderately effective, with 5-year survival rates ranging from 20% to 25% and the commonly used human liver cancer cell lines such as HepG2 [22] (Figures 3-5).
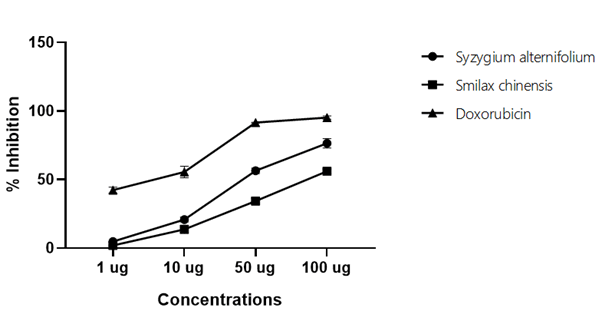
Figure 3: Anticancer activity of (% inhibition) of PANC-1 cell lines

Figure 4: Anticancer activity of (% inhibition) of BxPC-3 cell lines treated
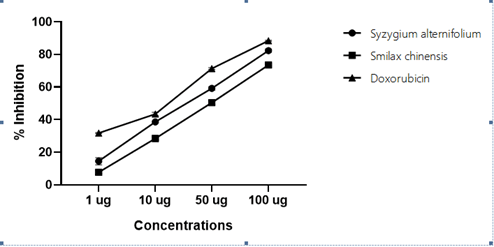
Figure 5: Anticancer activity of (% inhibition) of HepG2 cell lines treated
Both in vitro and in vivo experimentation involving cancer cell lines remains a convenient starting point for discovery and proof-of-concept studies. The study was extended to evaluate the in vitro anticancer activity of Syzygium alternifolium and Smilax chinensis by employing MTT assay in PANC 1, BxPC-3, and HepG2 cell lines representing both pancreatic and hepatic carcinomas (Tables 3-5).
Table 3: Anticancer activity of (% inhibition) of PANC-1 cell lines
| Concentrations (µg) | Syzygium alternifolium | Smilax chinensis | Doxorubicin |
|---|---|---|---|
| 1 | 4.86 ± 1.53 | 1.88 ± 0.34 | 42.29 ± 2.16 |
| 10 | 20.78 ± 1.77 | 13.73 ± 2.88 | 55.53 ± 4.20 |
| 50 | 56.38 ± 1.96 | 34.31 ± 2.08 | 91.51 ± 0.27 |
| 100 | 76.41 ± 3.44 | 56.06 ± 1.64 | 95.19 ± 1.26 |
| IC50 | 55.072 | 85.45 | 1.48 |
Table 4: Anticancer activity of (% inhibition) of BxPC-3 cell lines treated
| Concentrations (µg) | Syzygium alternifolium | Smilax chinensis | Doxorubicin |
|---|---|---|---|
| 1 | 15.91 ± 0.34 | 8.27 ± 0.09 | 27.99 ± 1.27 |
| 10 | 36.48 ± 1.16 | 15.09 ± 1.26 | 45.69 ± 1.64 |
| 50 | 61.87 ± 1.49 | 38.35 ± 0.66 | 70.20 ± 0.47 |
| 100 | 74.06 ± 0.76 | 65.60 ± 0.29 | 89.98 ± 0.50 |
| IC50 | 45.67 | 72.08 | 25.82 |
Table 5: Anticancer activity of (% inhibition) of HepG2 cell lines treated
| Concentrations (µg) | Syzygium alternifolium | Smilax chinensis | Doxorubicin |
|---|---|---|---|
| 1 | 14.50 ± 2.23 | 7.70 ± 0.69 | 31.77 ± 0.26 |
| 10 | 38.66 ± 0.54 | 28.43 ± 0.82 | 43.47 ± 1.09 |
| 50 | 59.21 ± 1.09 | 50.48 ± 1.12 | 71.38 ± 0.59 |
| 100 | 82.38 ± 0.98 | 73.56 ± 0.67 | 88.43 ± 0.30 |
| IC50 | 42.42 | 56.62 | 24.58 |
The treatment with Alcoholic Fruit extract of Syzygium Alternifolium (AFSA), Alcoholic leaf extract of Smilax Chinensis (ALSC), and doxorubicin showed inhibition of cancer cells in different cell line models. The order of potency of an inhibitory concentration of PANC 1, BxPC-3, and HepG2 cell lines was Doxorubucin>Syzygium alternifolium> Smilax chinensis. The activity might be due to the presence of active phytochemicals such as Hexadecanoic acid, Octadecenoic acid, and Cinnamic acid present in the AFSA and ALSC. Anticancer activities of cinnamic acid derivatives were reported by induction of apoptosis by irreversible DNA damage leading to cell death, and S-phase arrest. Apoptosis induction was exerted by the extract via alteration in mitochondrial membrane potential. The cytotoxic and genotoxic potential of cinnamic acid was found to be against the human melanoma cell line (HT-144) and human melanocyte cell line derived from blue nevus (NGM); also, against MTT assay on human cervical HeLa, ovarian SKOV-3, and breast MCF-7 cancer cell lines; oral squamous cell carcinoma (SCC25 ATCC CRL1628 cell lines), Human colorectal adenocarcinoma (LoVo, ATCC® CCL-229TM), human ovary adenocarcinoma (SkOV-3, ATCC® HTB-77TM), human lung non-small cell carcinoma (A549, ATCC® CCL-185TM), human mammary adenocarcinoma (MCF-7, ATCC® HTB-22TM), human pancreatic adenocarcinoma (AsPC-1, ATCC® CRL-1682TM) and human hepatocellular carcinoma (HepG-2, ATCC® HB-8065TM) cell lines with newly designed cinnamic acid hydrazides [23-26]. The Hexadecanoic acid acts as a potential cell growth inhibitor and cells were inhibited in the G0/ G1 phase; Hexadecanoic acid isolated from the Prunella vulgaris reported for HepG2, HT29, A549, MKN45, and HeLa cancer cell lines; against human colorectal carcinoma (HCT-116) cells; the anticancer actions as IC50 against human colorectal adenocarcinoma (Caco-2), mammary cancer cells (MCF-7), and human cervical carcinoma (HeLa) cells; MCF-7 (human breast cancer), HT-29 (human colon cancer), PC-3 and DU-145 (human prostate cancer) and CoN (human colon epithelial cells CCD 841) [27-31].
Conclusion
The reported phytochemicals namely Hexadecanoic acid, Octadecenoic acid, and Cinnamic acid present in the AFSA and ALSC were found to have in vitro anticancer activity against the PANC 1, BxPC-3, and HepG2 significantly. Hence, the study is planning to extend to identify the in vivo anticancer activity of Syzygium alternifolium (AFSA), an Alcoholic Leaf extract of Smilax chinensis (ALSC) using suitable animal models of pancreatic cancer.
Acknowledgement
None.
Conflict Of Interest
Authors have no conflict of interest to declare.
References
- A.P. Klein, Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors, Nat Rev Gastroenterol Hepatol, 18(2021):493â??502.
- F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J Clin, 68(2018):394â??424.
- S. Rossi, P. Baili, R. Capocaccia, M. Caldora, E. Carrani, et al. The EUROCARE-5 study on cancer survival in Europe 1999-2007: Database, quality checks and statistical analysis methods, Eur J Cancer,51(2015):2104â??2119.
- A. Kim, J. Ha, J. Kim, Y. Cho, J. Ahn, et al. Natural products for pancreatic cancer treatment: From traditional medicine to modern drug discovery, Nutrients, 13(2021):3801.
- L. Lin, P.S. Leung, Use of herbal medicines and natural products: An alternative approach to overcoming the apoptotic resistance of pancreatic cancer, Int J Cell Biol, 53(2014):224-236.
- Q. Yue, G. Gao, G. Zou, H. Yu, X. Zheng, Natural products as adjunctive treatment for pancreatic cancer: Recent trends and advancements, Biomed Res Int, (2017):8412508.
- M. Botta, S. Armaroli, D. Castagnolo, G. Fontana, P. Pera, et al. Synthesis and biological evaluation of new taxoids derived from 2-deacetoxytaxinine, J Bioorg Med Chem Lett, 15(2007):1579-1583.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, J Immunol Meth, 65(1983):55-63.
- K. Gopalakrishnan, R. Udayakumar, GC-MS analysis of phytocompounds of leaf and stem of marsilea quadrifolia (L), Int J Biochem Res Rev, 4(2014):517â??526.
- T. Starlin, P.S. Prabha, B.K.A. Thayakumar, V.K. Gopalakrishnan, Screening and GC-MS profiling of ethanolic extract of Tylophora pauciflora, Biomed Inform, 15(2019):425â??429.
- U. Naseem, Z. Muhammad, K.F. Ali, K. Shazeb, A review on general introduction to medicinal plants, its phytochemicals and role of heavy metal and inorganic constituents, Life Sci, 11(2014):520â??527.
- B. Helen, G. Haftom, B.T. Kald, Mariamawit, Ethiopian medicinal plants traditionally used for wound treatment: A systematic review, J Health Dev, 33(2019).
- R.I. Okoli, A.A. Turay, J.K. Mensah, A.O. Aigbe, Phytochemical and antimicrobial properties of four herbs from Edo state, Nigeria, Rep Opin, 1(2009):67â??73.
- K.K. Lakshmi, D. Akalanka, K. Satyavathi, P. Bhojaraju, GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC, Pharmacognosy Res, 6(2014):58â??61.
- Z. Li, Z. Zhang, L. Wu, H. Zhang, Z. Wang, Characterization of five kinds of wood vinegar obtained from agricultural and forestry wastes and identification of major antioxidants in wood vinegar, Chem Res Chinese U, 35(2019):12-20.
- S. Yilmaz, M. Sova, S. Ergun, Antimicrobial activity of trans-cinnamic acid and commonly used antibiotics against important fish pathogens and nonpathogenic isolates, J Appl Microbiol, 125(2018):1714â??1727.
- S.R. Wang, W. Yang, Y. Fan, W. Dehaen, Y. Li, et al. Design and synthesis of the novel oleanolic acid-cinnamic acid ester derivatives and glycyrrhetinic acid-cinnamic acid ester derivatives with cytotoxic properties, Bioorg Chem, 88(2019):1â??15
- S. Guo, Y. Zhen, Z. Zhu, G. Zhou, X. Zheng, Cinnamic acid rescues behavioral deficits in a mouse model of traumatic brain injury by targeting MiR-455-3p/HDAC2, Life Sci, 235(2019):1â??9.
- K. Liu, Q. Chen, Y. Liu, X. Zhou, X. Wang, Isolation and biological activities of decanal, linalool, valencene, and octanal from sweet orange oil, J Food Sci, 77(2012):C1156-61.
- S. Jones, X. Zhang, D.W. Parsons, J.C. Lin, R.J. Leary, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses, Science, 321(2008):1801â??1806
- Y.M. Hashim, D. Spitzer, S. Vangveravong, M.C. Hornick, G. Garg, Targeted pancreatic cancer therapy with the small molecule drug conjugate SW IV-134, 8(2014):956-967.
- T.M. Pawlik, E.K. Abdalla, C.C. Barnett, Feasibility of a randomized trial of extended lymphadenectomy for pancreatic cancer, Arch Surg, 140(2005):584â??589.
- E.L.O. Niero, G.M. Machado-Santelli, Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells, J Exp Clin Cancer Res, 32(2013):31.
- A. Bulakowska, J. Slawinski, R. Halasa, A. Hering, M. Gucwa, et al. An in vitro antimicrobial, anticancer and antioxidant activity of n-[(2-arylmethylthio)phenylsulfonyl] cinnamamide derivatives, Molecules, 30(2023):3087.
- S. Varadarajan, M. Narasimhan, T.M. Balaji, D.P. Chamundeeswari, D. Sakthisekaran, In vitro anticancer effects of cinnamomum verum J. presl, cinnamaldehyde, 4 hydroxycinnamic acid and eugenol on an oral squamous cell carcinoma cell line, J Contemp Dent Pract, 21(2020):1027â??1033.
- H. Mohamed, S.K. Bjelogrlic, P. Nevena, C. Ilija, B. Aleksandra, et al. Antimycobacterial and anticancer activity of newly designed cinnamic acid hydrazides with favorable toxicity profile, Arab J Chem, 15(2022):103532.
- B. Boobalan, P. Kantharaj, D. Sandhanasamy, S. Mohamad, S. Muthupandian, Evaluation of the anticancer potential of hexadecanoic acid from brown algae Turbinaria ornata on HTâ??29 colon cancer cells, J Mol Struct, 1235(2021):130229.
- Y.J. Hwang, E.J. Lee, H.R. Kim, K.A. Hwang. In vitro antioxidant and anticancer effects of solvent fractions from Prunella vulgaris var. lilacina, BMC Complement Altern Med. 13(2013):310.
- R. Lokesh, K. Kannabiran, Cytotoxic potential of n-hexadecanoic acid extracted from kigelia pinnata leaves, Asian J Cell Biol, 12(2017):20-27.
- A.J. Ahmed, F.O. Abdullah, K.K. Abdulrahman, Y. Galali, A.S. Sardar, GC-MS analysis of bioactive compounds in methanolic extracts of papaver decline and determination of its antioxidants and anticancer activities, J Food Qual, (2022).
- M. Mellado, M. Soto, A. Madrid, I. Montenegro, C. Jara-Gutierrez, et al. In vitro antioxidant and antiproliferative effect of the extracts of Ephedra chilensis K Presl aerial parts, BMC Complement Altern Med, (2019):19.
Copyright: © 2023 Jiten Mishra, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

