Research Article - Journal of Drug and Alcohol Research ( 2023) Volume 12, Issue 10
Nephroprotective Activity of Rutin in Diabetic Nephropathy Rat Model Targeting Nitric Oxide Pathway
Pathivada Manasa and Ganta Suhasin*Ganta Suhasin, Department of Pharmacology, GITAM School of Pharmacy, India, Email: sganta@gitem.edu
Received: 01-Nov-2023, Manuscript No. JDAR-23-122797 ; Editor assigned: 03-Nov-2023, Pre QC No. JDAR-23-122797 (PQ); Reviewed: 17-Nov-2023, QC No. JDAR-23-122797; Revised: 22-Nov-2023, Manuscript No. JDAR-23-122797 (R); Published: 29-Nov-2023, DOI: 10.4303/JDAR/236270
Abstract
The research conducted both In vitro and In vivo explored Rutin's impact on diabetic nephropathy, focusing on its effects on Nitric Oxide (NO) pathways in renal endothelial cells and in diabetic rats. In vitro, experiments using HRGECs revealed Rutin's ability to mitigate high glucose-induced cell death and reduced clearance of Bovine Serum Albumin on endothelial cells. Rutin's capacity for nitric oxide scavenging activity demonstrates its potential as an antioxidant. In the in vivo study, Rutin administration appeared to mitigate kidney damage induced by diabetes, as evidenced by reduced serum and urinary parameters associated with diabetic nephropathy. Moreover, the study observed a dose-dependent increase in nitrite/nitrate levels and collagen content in the kidneys. Histological abnormalities often refer to structural changes in tissues when examined under a microscope and high doses of Rutin might lead to an increase in the expression of eNOS in protective kidneys. This effect could be attributed to a decrease in the production of iNOS and nNOS protein levels. Rutin and L-Arginine hold promise as therapeutic avenues for addressing diabetic nephropathy, leveraging the nitric oxide pathway.
Keywords
Diabetic nephropathy; Diabetes mellitus; Reactive oxygen species; Streptazocine; Nicotinamide; Rutin; Nitric oxide pathway
Introduction
Diabetic Nephropathy (DN) is a highly complicated condition that affects people all over the world via a combination of environmental and genetic factors. Around 40% of diabetic people have DN [1]. By 2030 diabetes may be the 6th leading cause of mortality worldwide, as per the World Health Organization [2].
Nephropathy, Neuropathy, and Retinopathy are diabetic microvascular complications influenced by endothelial dysfunction. A gaseous lipophilic molecule named Nitric Oxide (NO) functions as a free radical and a gas transmitter, formed by the action of an enzyme Nitric oxide synthase on L-Citrulline. Nitric Oxide Synthases (NOS) have 3 different forms: Endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) [3]. Most of these endothelial functions rely on NO generated by endothelial cells’ by eNOS [4]. Increased NO generation may be the reason for the early hyper-filtration and microalbuminuria of diabetic nephropathy.
In contrast, the most of research indicates that advanced nephropathy is associated with proteinuria, worsening renal function, and hypertension, which has been associated with an increasing NO deficit. As a result, Nitric oxide can also be a good target for the treatment of diabetic nephropathy [5]. The purpose of the current research is to evaluate rutin’s influence on the nitric oxide pathway and its potential to prevent diabetic nephropathy. Based on the literature review, phytonutrients present in fruits and vegetables can protect the human body against reactive oxygen species. Natural antioxidants provide potential health benefits [6]. Flavonoids have considerable antioxidant capacities and have a preventative influence on oxidative stress-induced damage in several diseases such as cardiovascular disorders, diabetes, inflammation, and heaptoxicity [7]. Phytochemicals are enticing alternatives since they are many, affordable, and have fewer disadvantages than pharmaceutical substances [8].
Rutin is a glycoside that combines the flavanol quercetin with the disaccharide rutinose and it is a citrus flavonoid found in many plants, especially citrus fruits [9]. It is proven to have many Pharmacological activities including cardioprotective, hepato-protective, anti-carcinogenic, and anticonvulsant properties [10-13]. The review suggested that Rutin also possesses anti-diabetic activity [14] (Figure 1).
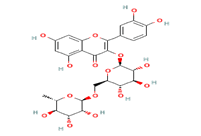
Figure 1: Structure of Rutoside, Quercetin-3-O-Rutinoside or Sophorin (Rutin)
Material and Methods
Chemicals
Sisco Research Laboratories Pvt. Ltd. Mumbai: Captopril (C. No-211875), L-NAME (C. No-29299090), L-Arginine (C. No-29224990), Rutin (C. No-250249-75-3). Sigma Aldrich Pvt.Ltd. USA: Nicotinamide (NCT) and Streptazocine (STZ). All chemicals used were of analytical grade.
In vitro evaluation
High hyperglycemia (Hg)-induced cytotoxicity/ apoptosis of HUMAN RENAL Glomerular Endothelial Cells (HRGECs): HRGECs are endothelial cells of capillaries that are susceptible to direct malfunction mortality in response to elevated blood glucose levels.
Cell proliferation assay: 3-[4,5-dimethylthiazol-2-yl]- 2,5-diphenyl tetrazolium bromide (MTT) assay is used to measure cellular metabolic activity as an indicator of cell viability, proliferation, and cytotoxicity. The MTT Assay was used to assess the attenuation activity of rutin against HG-induced apoptosis in HRGECs by Y. Bendale et al. (2017) method absorbance was measured at 570 nm [15].
Endothelial cell permeability: Endothelial permeability was assessed by measuring albumin diffusion across HRGECs monolayer according to Yuyan Fan et al. (2019) method [16]. Monolayer cells were planted on the Transwell inserts (3422, coring, USA) and incubated with Phosphate buffer saline which contains 0.035% trypan blue and 0.8% BSA at 37°C for one hour. Absorbance at 590 nm was measured from the lower well fluids, and the percentage clearance of bovine serum albumin was used to assess the permeability of the HRGECs monolayer.
Nitric oxide scavenging activity: To perform NO scavenging activity, the Rutin sample was combined with two milliliters of sodium nitroprusside (10 mM) in varying doses (0.2 mg/ml-0.8 mg/ml) and the standard ascorbic acid stored at 4°C. Griess reagent was prepared and 0.5 ml of the Griess reagent was added to the mixture, it was incubated for an extra five minutes at 25°C. The absorbance at 546 nm was determined [17]. The equation below is used to calculate the degree of nitric oxide scavenging activity:
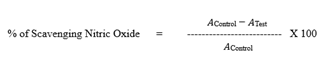
Evaluation of nephroprotective activity of rutin-in vivo study
Animals and diet: Swiss albino mice and male Sprague Dawley rats were purchased from Vyas Labs in Hyderabad. For one week, they were acclimated in the animal house. VRK Nutritional Pvt. Ltd., Hyderabad provided the dietary supplements. The study protocol received the following approval number from the Institutional Animal Ethics Committee (IAEC) of the GITAM School of Pharmacy: IAEC/GIP-128/PM- RS/Approved/2/2021.
Acute toxicity study: According to OECD guideline 423 limit test, three female mice received Rutin 2000 mg/kg, b.w., orally after being deprived of food the previous night, and they were monitored for 14 days for any indicators of mortality and toxic effects. For further in-vivo testing, 1/20th and 1/40th doses of the maximum dosage were used.
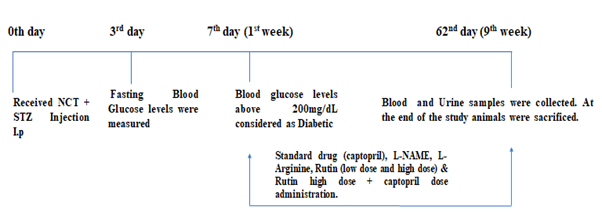
Induction of diabetic nephropathy: After one week of acclimatization, Sprague Dawley rats were treated with a dose of Streptazocine (STZ) (55mg/kg, b.w., i.p) 15 minutes after Nicotinamide (100 mg/kg, b.w, i.p) to induce Diabetes Mellitus (DM). To reduce the death rate, animals were given 5% glucose after 6 hours of STZ to 24 hours. Rats were confirmed as diabetic on day 7 whose fasting blood glucose levels were 200 mg/dL or above (Figure 2).
Figure 2: Evaluation of nephroprotective activity of Rutin-in vivo study
In 1% sodium Carboxy Methyl Cellulose (CMC), rutin suspension was prepared. Distilled water was used to dissolve the standard medication (captopril), L-NAME, and L-Arginine.
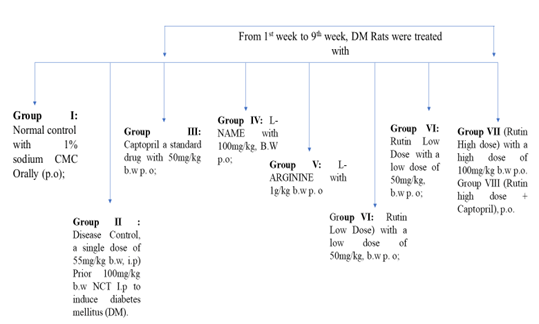
48 animals male Sprague Dawley rats’ Body weights were assessed after one week of acclimatization and also divided into 8 groups which contained 6 animals each (Figure 3).
Figure 3: Evaluation of nephroprotective activity of Rutin-in vivo study
Determination of biological parameters
A digital balance was used every week to weigh each animal’s Body weight.
Serum parameters: Blood samples (1 ml) were collected through the retro-orbital puncture after the 4th and 9th weeks to determine Serum Creatinine (SC) (Alkaline Picrate Kinetic), blood urea nitrogen (BUN) (Urease, Spectrophotometry), blood glucose levels (BGL) (one touch glucometer) [18,19].
Urine parameters: A metabolic cage was used to collect 24-hour urine samples for the determination of Albumin (AB) (Tetra Bromophenol Blue) and total protein (TP) (Pyrogallol Red) [20-22].
Kidney tissue parameters
Kidney Fractional Weight (KFW): Animals were euthanized with a high dosage of anesthesia at the end (9th week), and kidneys were isolated, washed, blotted in filter paper, weighed, and calculated with the below formula,
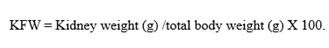
Lipid peroxidation
Tissue homogenate: To prepare a 10% kidney homogenate, Phosphate-Buffered Saline (PBS) with a pH of 7.4 (0.1 M) was used to homogenize each rat kidney.
Methodology: In the centrifuge tube, 0.5 mL of kidney homogenate, 3 milliliters of 1% phosphoric acid, and 1 mL of 0.6% of Thio barbituric acid were mixed and tended to incubate for 45 minutes in a water bath under boiling. Four milliliters of n-butanol were added after cooling and then subjected to vortexing for a minute followed by centrifugation at 2000 rpm for 20 minutes. The separated layer was collected in the fresh tube and then, subjected to a spectrophotometer to study the absorbance at 532 nanometers [23].
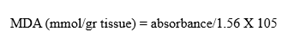
NO3-/NO2- levels: Serum, urine, and kidney NO3-/NO2- levels were estimated by the Kamiya Biomedical company kit procedure using Griess reagent [20].
Renal collagen content: Hydroxyproline content in renal cortical collagen was evaluated. Kidney slices were stained with Masson’s Trichrome, Van Gieson, and Verhoeff Van Gieson stains to reveal collagen fibers. The collagen content of histology slides was determined using Adobe Photoshop and compared to the results of three different histological stains [24].
Histological studies: In the saline, kidneys were rinsed and immersed in a 10% formalin solution for 2 days dehydrated in 50%-100% ethanol, and embedded in paraffin wax. 5 μm thickness slices were produced using a microtome and stained for microscopic examination with eosin and hematoxylin dyes and the prepared slides were studied under 400x magnification.
Western blotting: In western blot analysis, Protein levels were detected via antibodies against eNOS, iNOS, and nNOS in kidney homogenates. A chemiluminescence detection kit was used for the chemiluminescence detection process according to Khamaisi M, et al. (2005) [25].
Statistical analysis
The program Graph Pad Prism-8.0, is used for the evaluation of data. n=6 ± SEM. ### p<0.001 compared to a normal control group (CG); ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05 compared to the disease control, analyzed with one-way ANOVA paired with Tukey’s various analysis check.
Results and Discussion
Cell cytotoxicity assay
The results of the cell cytotoxicity assay revealed incubation of HRGEC cells in the High Glucose increased the cell cytotoxicity compared to the Control cells, attenuated by using rutin. L-NAME with the addition of rutin reduced the protective effect of rutin on HG-induced cytotoxicity. The absorbance values were examined and demonstrated as a graphical representation and photo-micrographic sections summarized in Figure 4.
Figure 4: Cell cytotoxicity assay
Endothelial cell permeability
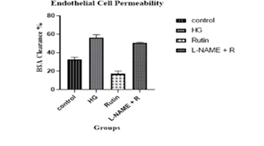
Diabetes mellitus impairs endothelial cell permeability, which may be exacerbated by high extracellular glucose concentrations. Under typical physiological conditions, the endothelium barrier sustains a minimum and selective permeability to fluid and molecules. The disruption of the endothelial barrier occurs in high glucose levels increasing the cell permeability thus it increases the Bovine Serum Albumin (BSA) clearance %. The findings from the endothelial cell permeability incubating HRGEC cells in the high glucose elevated BSA clearance % compared to the control cells, this was counteracted by rutin. L-NAME with rutin reduced the effect of rutin in HGenhanced HRGECs cell permeability. The Bovine Serum Albumin (BSA) clearance % values were examined and demonstrated in Figure 5.
Figure 5: Cell cytotoxicity assay
Nitric oxide scavenging activity
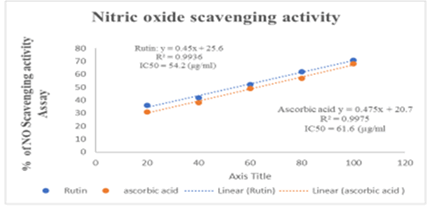
To test Rutin’s ability to scavenge NO radicals, the NO Radical Scavenging Assay technique was employed. Griess reagent forms a purple azo product when it combines with nitrite in samples, whose absorbance was measured at 546 nm. In an in vitro study, Rutin has shown dose-dependent NO scavenging activity. IC50 values of Rutin and ascorbic acid were found at 54.2 μg/ml and 61.6 μg/ml. The results are summarized in Figure 6.
Figure 6: Journa-Drug-Alcohol-Research-Nitricl-23-12-10-270-g006
Body weight
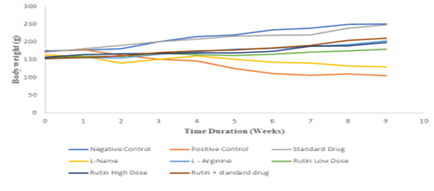
Body weight of Disease control significantly reduced when compared with normal control. Treatment of Captopril, L-Arginine, Rutin+Captopril, and Rutin’s high dose restored the body weight during 9 weeks of the study when compared with disease control. The results are summarized in Figure 7.
Fasting blood glucose levels
On the 3rd and 7th days following the STZ injection, fasting blood glucose levels were measured. Rats having fasting blood glucose levels of greater than 200 mg/dl on the seventh day were confirmed as diabetic. From the 1st to the 9th week of the trial, DM rats were treated with Rutin high dose, rutin high dose with captopril, L-Arginine, and captopril treatment reduced the blood glucose levels in rat diabetic nephropathy model more significantly. The results are summarized in Table 1.
Figure 7: Body weight during 9 weeks of study
Table 1: Serum parameters
| Groups | BGL (mg/dL) | BUN (mg/dL) | SR (mg/dL) |
|---|---|---|---|
| Control | 103 ± 4.3 | 15 ± 0.6 | 0.38 ± 0.06 |
| Disease control | 427 ± 9.4#### | 29 ± 2.9### | 0.8 ± 0.03## |
| Captopril | 235 ± 26*** | 18 ± 0.3** | 0.2 ± 0.028*** |
| L-Name | 391±31ns | 25 ± 1.3ns | 0.4 ± 0.04ns |
| L-Arginine | 223 ± 59*** | 13 ± 0.9*** | 0.29 ± 0.12*** |
| Rutin low dose | 308 ± 26* | 19 ± 1.97* | 0.33 ± 0.10* |
| Rutin high dose | 118 ± 3.6**** | 12 ± 0.3*** | 0.25 ± 0.11*** |
| Rutin+Captopril | 255 ± 9** | 21 ± 1.3* | 0.36 ± 0.07* |
| BGL-Blood Glucose Levels; BUN-Blood Urea Nitrogen; SR-Serum Creatinine | |||
Blood Urea Nitrogen (BUN)
BUN is a typical biomarker used to assess kidney function, and BUN levels are inversely linked to renal function. When compared to the disease control, Captopril, l-arginine, and Rutin high dose showed a substantial reduction in Blood urea nitrogen.
Serum Creatinine (SC)
Creatinine is present in serum, plasma, and urine and is being excreted by glomerular filtration at a consistent rate. It serves as a more dependable marker of renal function compared to BUN because it is less influenced by other factors such as diet and hydration. When compared to the disease control, Captopril, l-arginine, and Rutin high dose showed a significant reduction in Blood urea nitrogen.
Albumin and total protein
Albuminuria, or having too much albumin in the urine, is a symptom of renal impairment. Proteinuria is elevated protein in the urine. Significant quantities of protein cannot flow through the filters of healthy kidneys. When compared to the disease control the amount of urine albumin and proteins significantly decreased in l-arginine, rutin high dose. The results are summarized in Table 2.
Table 2: Urinary parameters
| Groups | AB (mg/dL) | TP (mg/dL) |
|---|---|---|
| Control | 13 ± 0.7 | 12 ± 0.9 |
| Disease control | 30 ± 1.1#### | 25 ± 0.8### |
| Captopril | 23 ± 0.6** | 17 ± 2.8* |
| L-Name | 25 ± 1.09ns | 19 ± 1.4ns |
| L-Arginine | 22 ± 2.1** | 15 ± 1.2** |
| Rutin low dose | 23 ± 1.2* | 17 ± 1.5* |
| Rutin high dose | 21 ± 2.0** | 14 ± 1.2** |
| Rutin+Captopril | 24 ± 1.2* | 17 ± 1.3* |
| AB-Albumin; TP-Total Protein | ||
Kidney fractional weight
Animals were sacrificed further to study and kidney fractional weight was measured. When compared to the disease control the Kidney fractional weight significantly decreased in the captopril, L-arginine, and rutin high-dose treated groups. The results are summarized in Table 3.
Table 3: Tissue parameters
| Groups | KFW (g) | MDA levels (mm/l) |
|---|---|---|
| Control | 1.8 ± 0.03 | 69 ± 2.0 |
| Disease control | 2.29 ± 0.1 | 126 ± 11### |
| Captopril | 1.5 ± 0.19*** | 68 ± 1.4*** |
| L-Name | 2.0 ± 0.001ns | 103 ± 14ns |
| L-Arginine | 1.5 ± 0.21*** | 73 ± 2.3** |
| Rutin low dose | 1.68 ± 0.21* | 80 ± 10* |
| Rutin high dose | 1.09 ± 0.004**** | 70 ± 1.3** |
| Rutin+Captopril | 1.7 ± 0.2* | 83 ± 10* |
| KFW-Kidney Fractional Weight; MDA Levels-Malondialdehyde Levels | ||
Lipid peroxidation
Lipid peroxidation reveals a detrimental process, leading to extensive morbidities in cell membrane functions and structure which tends to cell injury. This is measured by investigating secondary metabolites indirectly like reactive low molecular weight Aldehyde-Malondialdehyde (MDA). The groups were treated with captopril, L-arginine, and rutin high-dose and MDA levels were significantly lower when compared to the disease-control group. The observations are summarized in Table 3.
Blood, urinary, and total kidney NO3-/NO2- levels
Additionally, studies have shown a clear correlation between Vascular Endothelial Dysfunction (VED) and DN, and it has been discovered that a reduction in serum NO3-/NO2- concentration is an indication of VED. The serum, urine, and total kidney concentration of nitrate/nitrite levels increased with L-arginine, and Rutin dose-dependent. The findings are summarized in Table 4.
Table 4: NO3-/NO2- levels
| Groups | Blood (NO3-/NO2-) levels | Urinary (NO3-/NO2-) levels (µm) | Total kidney (NO3-/NO2-) levels (µm) |
|---|---|---|---|
| Control | 33 ± 0.3 | 2.14 ± 0.2 | 2.8 ± 0.02 |
| Disease control | 12 ± 0.1#### | 1.3 ± 0.16# | 1.6 ± 0.09#### |
| Captopril | 22 ± 1.6*** | 2.3 ± 0.16** | 2.2 ± 0.1** |
| L-Name | 11 ± 0.4ns | 1.9 ± 0.07ns | 1.8 ± 0.21ns |
| L-Arginine | 21 ± 1.03** | 2.5 ± 0.07*** | 2.2 ± 0.18** |
| Rutin low dose | 18 ± 2.4* | 2.3 ± 0.2** | 2.1 ± 0.01* |
| Rutin high dose | 25 ± 2.1*** | 2.4 ± 0.16*** | 2.4 ± 0.02*** |
| Rutin+Captopril | 19 ± 1.9* | 2.2 ± 0.002* | 2 ± 0.02* |
| NO3-/NO2- levels-Nitrate and Nitrite levels | |||
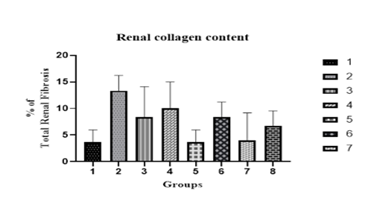
Renal collagen content
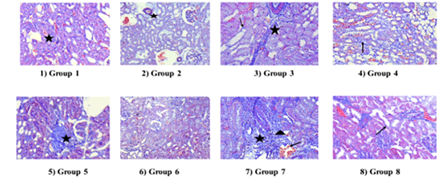
Renal fibrosis is one of the vital factors in both aging and pathological conditions which takes part in evaluating the collagen content concentration (collagen content/dry weight tissue), and subtypes from renal tissues. DN model of the renal collagen content increased significantly. The proportion of renal fibrosis decreased dose-dependent with Rutin. The % of renal fibrosis was measured graphically and Masson’s Trichrome stains are summarized in Figures 8 and 9.
Figure 8: Graphical representation of total renal collagen content
Figure 9: Photomicrograph of renal collagen content
The Normal control group showed normal architecture of the kidney. Disease control showed severe intertubular fibrous tissue proliferation in the cortex and medulla and severe congestion of intertubular capillaries. Rats treated with captopril showed very mild intertubular fibrous tissue proliferation in the cortex and medulla. Nitric oxide synthase inhibitor, L-NAME showed severe intertubular fibrous tissue proliferation and congestion of intertubular capillaries and Precursor, L-Arginine with moderate fibrous tissue proliferation in the cortex and medulla. Rutin with two doses showed mild fibrous tissue proliferation. A combination of Rutin with captopril showed moderate tissue proliferation with Masson’s Trichrome stain 100X.
Histopathological studies
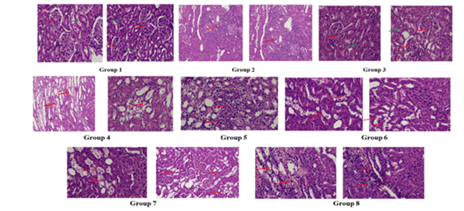
In histological examinations, normal kidney tubule morphology in the cortical region and glomerulus. Disease control histological observation showed moderate tubular nephritis: Inflammation of the tubules and interstices with many foci and lymphocyte infiltration. Rats treated with captopril showed a brush border of the proximal convoluted tubules as well as basal laminae of tubules. In L-NAME, the cortical region’s proximal convoluted tubules had mild tubular degeneration and significant brush border. Whereas, collecting channels in the vicinity of the renal pelvis showed foci of cystic dilatation and cystic tubular degeneration in L-Arginine, Visceral epithelial cell foot in one area underwent a microvillous metamorphosis in Rutin Low Dose, and the glomerular basement membrane was normal; there was no significant mesangial cell growth or mesangial matrix deposition. Mesangial cell apoptosis was shown in Rutin high dose, and in Rutin high dose with captopril showed mild tubular/interstitial irritation with inflammatory cell infiltration (Figure 10).
Figure 10: Histopathological studies, Group 1-Normal control; Group 2-disease control; Group 3- Captopril; Group 4-L-NAME; Group 5-L-Arginine; Group 6-Rutin Low Dose; Group 7-Rutin High Dose; Group 8-Rutin high Dose+Captopril
Western blot
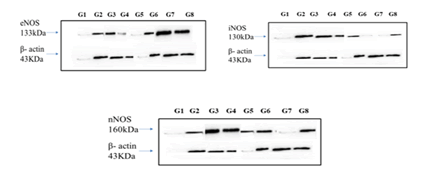
In our study, no significant difference in eNOS expression was observed in the disease group when compared to the control group; a significant increase compared with the disease group was detected in Rutin high dose and a combination of rutin high dose with captopril. The reduction in eNOS-derived NO in diabetic nephropathy contributes significantly to endothelial dysfunction, inflammation, oxidative stress, and impaired vascular and renal function. Therapeutic strategies aimed at enhancing eNOS activity have been explored as potential approaches to mitigate diabetic kidney disease progression by restoring vascular function and reducing renal damage [26]. The current study observed a significant increase in iNOS expression in the disease group concerning the normal control, rutin in both doses attenuated this increase. Rutin may have anti-inflammatory properties because of its scavenger properties and inhibition of iNOS expression. Many cell types are activated by cytokines like tumor necrosis factor-α, which trigger iNOS and produce copious amounts of NO, whose role in renal pathophysiology is still up for controversy. Several studies have indicated that a medium level of NO produced through eNOS might be advantageous for its vasodilator action, but high levels of NO produced by iNOS may result in peroxynitrite anion, an effective oxidant that can cause pathological kidney failure conditions [27]. Also, a significant difference in nNOS expression was observed in the disease group concerning controls; whereas, captopril, L-Arginine, and a combination of rutin high dose with captopril-treated rats showed an increase when compared to the disease control. Diabetes often leads to increased oxidative stress. nNOSproduced NO can interact with Reactive Oxygen Species (ROS), forming Reactive Nitrogen Species (RNS) that can further contribute to oxidative stress within the kidney, potentially exacerbating renal damage [28]. Western blot analysis is commonly used in molecular biology to detect specific proteins in a sample. In this case, the study seems to suggest that Rutin at higher doses might modulate the expression levels of different nitric oxide synthases, potentially influencing kidney protection through the regulation of these proteins (Figure 11).
Figure 11: Western blot analysis: ÃÂ??-Actin was used for the loading control as well as the control for eNOS, nNOS and iNOS protein levels. G1-Normal control; G2-disease control; G3-Captopril; G4-L-NAME; G5-L-Arginine; G6-Rutin Low Dose; G7-Rutin High Dose; G8-Rutin high dose+Captopril
Discussion
Various factors are responsible for Endothelial Cell Dysfunction (ECD) which include, elevated blood pressure, maximum cholesterol levels, hyperglycemia, and other genetic factors [4]. Diabetes is a long-term metabolic disease that causes uncontrolled hyperglycemia because of a lack of insulin, insulin insufficiency, or resistance which leads to ECD. Prominent symptoms of diabetes-mediated abnormalities are indicated for diabetic retinopathy, nephropathy, neuropathy, peripheral artery disease, heart disease, stroke, and erectile dysfunction [29].
Numerous diseases are treated in traditional practice with the help of crude drugs and medicinal plants. The use of natural biomolecules in the research and development of new drugs has several distinct benefits. They are chemical novelties that can produce lead drug candidates for challenging targets. The flavonoid rutin generally appears in buckwheat, onions, apples, tea, and red wine, and helps to scavenge Reactive Oxygen Species (ROS) [30]. It also serves as a chelating agent and terminator for metal ions that can cause lipid peroxidation [31]. The antioxidant capabilities of Rutin may be the cause of these benefits. Rutin may have benefits for the heart, the reduction of inflammation, asthma, and cholesterol, the prevention of cancer, and the preservation of neurons [32]. Previous research in phorbol myristate acetate/ionomycin-induced EL4 murine T-lymphoma cells has demonstrated that rutin modulates immune responses by suppressing IL-4, inhibiting nuclear factor of activated T cells and GATA3, and lowering T helper-2 cell signaling (p-STAT6) [33,34].
One important regulatory molecule that has a wide range of effects on metabolism, blood vessels, and cells is Nitric Oxide (NO). Since insulin regulates NO Synthase (NOS) activation through the Akt pathway, controlling NO metabolism is especially crucial in type 2 diabetes. Thus, insulin resistance that also affects the vascular response may result in disruptions of NO generation [35]. Type 2 diabetes is associated with impaired NO metabolism, particularly when nephropathy is present [36]. Under physiological and pathological circumstances, NOS (neuronal, inducible, and endothelial) produces NO, an important inflammatory mediator [37]. In addition, it plays a critical role as a mediator in the inflammatory process. Enhanced NO generation and iNOS expression are responsible for the cytotoxic effect.
NO is thought to be implicated in both the cause of type 2 diabetes and the defective insulin-mediated stimulation of blood flow [38]. In diabetics, abnormal NO production affects renal structure and functions. Reduced eNOS expression, substrate deficiency for nitric oxide synthases because of enhanced arginase activity and minimized L-arginine availability, eNOS decoupling due to elevated ROS, oxidation of tetrahydrobiopterin (BH4), depletion of L-arginine, and accumulation of methylarginines which led to superoxide anion instead of NO production caused a reduction in nitric oxide levels in diseased conditions [39].
L-NAME indeed has a notable impact on Nitric Oxide (NO) availability, which affects various physiological functions, including blood pressure regulation. Its inhibition of Nitric Oxide Synthase (NOS) decreases NO production, leading to vasoconstriction and increased blood pressure. This effect is often associated with the progression of conditions like Diabetic Kidney Disease [40]. Olga Pechanova, et al., showed the intricate mechanisms involved in this process [41]. Their findings indicate that increased expression of endothelial NOS (eNOS) may contribute to heightened NOS activity in peripheral tissues after prolonged L-NAME treatment. The multifaceted nature of L-NAME’s impact on NOS activity, blood pressure regulation, and tissuespecific effects underscores the complexity of its role in physiological processes and highlights the need for further research to fully comprehend its mechanisms and potential therapeutic interventions.
L-NAME, a NOS inhibitor worsened structural damage to the renal interstitial tissue. The renal injury pattern showed modest glomerular lesions, and tubulointerstitial severe damage, even though the ultimate stage of glomerulosclerosis was not achieved and the L-NAME group was shown the kidney damage as similar to the diseased condition. So, it can also be used for the induction of Nephropathy.
A beneficial impact of acute and chronic L-arginine enrichment on endothelial-derived nitric oxide production and endothelial function has been revealed in several studies [42]. Consuming L-Arginine supplementation in the treatment of diabetic nephropathy gives a better effect than the diseased group. L-arginine restored the body weight and reduced blood glucose levels, BUN, serum creatinine, urinary albumin, and total protein. Reduced kidney fractional weight and MDA levels.
The selected natural biomolecule of Rutin high dose (100 mg/kg., B.W) showed significant efficacy in Diabetic kidney Rats. Moreover, ACE inhibitors i.e., Captopril, and also with a combination of Rutin showed a significant impact on kidney damage. Captopril preserved vascular endothelium-dependent relaxation.
Conclusion
Overall, the research highlights the potential of targeting the nitric oxide synthase pathway as a strategy for managing diabetic nephropathy, with Rutin and L-arginine emerging as potential therapeutic options due to their impact on this pathway.
Acknowledgement
I would like to express my special thanks to Dr. Swathi Bora, M.V.Sc., Ph.D., Assistant Professor, Dept. of Veterinary Pathology, College of Veterinary Science, Rajendra Nagar, Hyderabad-30 for conducting kidney collagen content and histopathological studies and also for JEEVA LIFE SCIENCES, Plot No: 98, Shanthi Nagar, Hyderabad, Telangana-39 for helping us in in vitro studies.
Conflict Of Interest
Authors have no conflict of interest to declare.
References
- R.Z. Alicic, M.T. Rooney, K.R. Tuttle, Diabetic kidney disease: Challenges, progress, and possibilities, CJASN, 12(2017):2032-2045.
- M.A.B Khan, M.J. Hashim, J.K King, R.D Govender, H. Mustafa, et al. Epidemiology of type 2 diabetes-global burden of disease and forecasted trends, J Epidemiol Glob Health, 10(2019):107.
- B.S. Dellamea, C.B. Leitao, R. Friedman, L.H. Canani, Nitric oxide system and diabetic nephropathy, Diabetol Metab Syndr, (2014):6.
- G.K. Kolluru, S.C. Bir, C.G. Kevil, Endothelial dysfunction, and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing, Int J Vasc Med, (2012):1-30.
- V. Vallon, R. Komers, Pathophysiology of the diabetic kidney, Compr Physiol, (2011):1175-232.
- Y.J. Zhang, R.Y. Gan, S. Li, Y. Zhou, A.N. Li, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases, Mol, 20(2015):21138-56.
- A.N. Panche, A.D. Diwan, S.R. Chandra, Flavonoids: An overview, J Nutr Sci, 5(2016):e47.
- M.A. Ashraf, Phytochemicals as potential anticancer drugs: Time to ponder natureâ??s bounty, Biomed Res Int, (2020):1-7.
- A. Ganeshpurkar, A.K. Saluja, The pharmacological potential of rutin, Saudi Pharm J, 25(2017):149-64.
- A. Ziaee, F. Zamansoltani, M. Nassiri-Asl, E. Abbasi, Effects of rutin on lipid profile in hypercholesterolemia rats, Basic Clin Pharmacol Toxicol, 104(2009):253-8.
- R. Domitrovic, H. Jakovac, V. Vasiljev Marchesi, S. Vladimir-Knezevic, O. Cvijanovic, et al. Differential hepatoprotective mechanisms of rutin and quercetin in CCl4-intoxicated BALB/CN mice, Acta Pharmacol Sin, 33(2012):1260-70.
- S.S. Choi, H.R. Park, K.A. Lee, A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation, Antioxid, 10(2021):1696.
- N.A. Al-Dhabi, M. Valan Arasu, C.H. Park, S.U. Park, An up-to-date review of rutin and its biological and pharmacological activities, EXCLI J, (2015).
- A. Ghorbani, Mechanisms of antidiabetic effects of flavonoid rutin, Biomed Pharmacother, 96(2017):305-12.
- Y. Bendale, V. Bendale, S. Paul, Evaluation of the cytotoxic activity of platinum nanoparticles against normal and cancer cells and its anticancer potential through induction of apoptosis, Integr Med Res, 6(2017):141-8.
- J.A. Cooper, P.J. Del Vecchio, F.L. Minnear, K.E. Burhop, W.M. Selig, et al. Measurement of albumin permeability across endothelial monolayers in vitro, J Appl Physiol, 62(1987):1076-83.
- J. Benevides Bahiense, F.M. Marques, M.M. Figueira, T.S. Vargas, T.P. Kondratyuk, et al. Potential anti-inflammatory, antioxidant and antimicrobial activities of Sambucus australis, Pharm Biol, 55(2017):991-7.
- M. Peake, M. Whiting, Measurement of serum creatinine-current status and future goals, Clin Biochem Rev, 27(2006):173-84.
- J.F. Richards, R.V. Smith, R.E, Wilcox, Comparison of two spectrophotometric assays for blood urea nitrogen: Influence of apomorphine, Microchem J, 29(1984):49-55.
- Kamiya Biomedical Company, In vitro nitric oxide (nitrite/nitrate) assay kit (colorimetric) cat. no. KT-943, Nat Med, (1998).
- M.J. Pugia, J.A. Lott, J.A. Profitt, T.K. Cast, High-sensitivity dye binding assay for albumin in urine, J Clin Lab Anal, 13(1999):180-7.
- S. Behr, C. Trumel, F. Palanche, J.P. Braun, Assessment of a pyrogallol red technique for total protein measurement in the cerebrospinal fluid of dogs, J Small Anim Pract, 44(2003):530-3.
- K. Bellassoued, A. Ben Hsouna, K. Athmouni, J. van Pelt, F. Makni Ayadi, et al. Protective effects of Mentha piperita L. leaf essential oil against CCl4 induced hepatic oxidative damage and renal failure in rats, Lipids Health Dis, 17(2018):9.
- O. Abbadi, Measuring collagen concentration in histology slides of mice skin using the adobe® photoshop® software, 2019.
- M. Khamaisi, S. Keynan, M. Bursztyn, R. Dahan, E. Reinhartz, et al. Role of renal nitric oxide synthase in diabetic kidney disease during the chronic phase of diabetes, Nephron Physiol, 102(2006):72-80.
- S.S. Badal, F.R. Danesh, Strategies to reverse endothelial dysfunction in diabetic nephropathy, Kidney Int, 82(2012):1151-4.
- U. Forstermann, H. Li, Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling, Br J Pharmacol, 164(2011):213-23.
- I. Afanas'ev, Signaling of reactive oxygen and nitrogen species in diabetes mellitus, Oxid Med Cell Longev, (2010):3(6);361-73.
- W.T. Cade, Diabetes-related microvascular and macrovascular diseases in the physical therapy setting, Phys Ther, 88(2008):1322-35.
- A.V. Anand David, R. Arulmoli, S. Parasuraman, Overviews of biological importance of quercetin: A bioactive flavonoid, Pharmacogn Rev, 10(2016):84-89.
- S.J. Flora, V. Pachauri, Chelation in metal intoxication, Int J Environ Res Public Health, 7(2010):2745-88.
- A.B. Enogieru, W. Haylett, D.C. Hiss, S. Bardin, O.E. Ekpo, Rutin as a potent antioxidant: Implications for neurodegenerative disorders, Oxid Med Cell Longev., 27(2018):6241017.
- C. Stremmel, E.A. Greenfield, E. Howard, G. J. Freeman, V. K. Kuchroo, B7-2 expressed on EL4 lymphoma suppresses antitumor immunity by an interleukin 4-dependent mechanism, J Exp Med, 189(1999):919-30.
- E. Maier, A. Duschl, J. Horejs-Hoeck, STAT6-dependent and independent mechanisms in Th2 polarization, Eur J Immunol, 42(2012):2827-33.
- N. Tuteja, M. Chandra, R. Tuteja, M.K. Misra, Nitric oxide as a unique bioactive signaling messenger in physiology and pathophysiology, J Biomed Biotechnol, (2004):227-237.
- P.K. Dabla, Renal function in diabetic nephropathy, World J Diabetes, 15(2010):48-56.
- E.D. Costa, B.A. Rezende, S.F. Cortes, V.S. Lemos, Neuronal nitric oxide synthase in vascular physiology and diseases, Front Physiol, 7(2016):206.
- P. Tessari, D. Cecchet, A. Cosma, M. Vettore, A. Coracina, et al. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy, J Diabetes, 59(2010):2152-9.
- V. Suresh, A. Reddy, Dysregulation of nitric oxide synthases during early and late pathophysiological conditions of diabetes mellitus leads to amassing of microvascular impediment, J Diabetes Metab Disord, 20(2021):989-1002.
- R. Corremans, P.C. D'Haese, B.A. Vervaet, A. Verhulst, L-NAME administration enhances diabetic kidney disease development in an STZ/NAD rat model, Int J Mol Sci, 22(2021):12767.
- O. Pechanova, S. Vrankova, M. Cebova, Chronic L-name-treatment produces hypertension by different mechanisms in peripheral tissues and brain: Role of central eNOS, Pathophysiol, 27(2020):46-54.
- J. Gambardella, W. Khondkar, M.B. Morelli, X. Wang, G. Santulli, et al. Arginine and endothelial function, Biomedicines, 8(2020):277.
Copyright: © 2023 Pathivada Manasa, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.