Research Article: Journal of Drug and Alcohol Research (2024) Volume 13, Issue 1
Patent Pools: Opportunities for Innovation and Access to Essential Medicine for Under-Served Populations in the 21st Century
Mohd Kaif1, Akanksha Verma1*, Mukesh Kumar Dudi1, Sachin Yadav2 and Deepak Kumar Chauhan32Research Scholar, University of Galway, Ireland
3Department of Law, Central University of Punjab, India
Akanksha Verma, Research Scholar, Central University of Punjab, India, Email: akankshaverma.law@gmail.com
Received: 03-Jan-2024, Manuscript No. JDAR-24-125086; Editor assigned: 05-Jan-2024, Pre QC No. JDAR-24-125086 (PQ); Reviewed: 19-Jan-2024, QC No. JDAR-24-125086; Revised: 24-Jan-2024, Manuscript No. JDAR-24-125086 (R); Published: 31-Jan-2024, DOI: 10.4303/JDAR/236277
Abstract
Patent pools have been employed in a range of industries for a variety of reasons, and they have resulted in a number of benefits for patent holders as well as the industry at large. As a result, using patent pools to encourage access to technology is not a novel concept. Patent pools lessen the risks associated with implementing patented technologies while also reducing the time and money associated with individual licence negotiations. Patent pools have historically been employed successfully in information technology, consumer electronics, and other industries with a large number of patents. However, they may not be as suitable for the pharmaceutical and biotechnology industries. The purpose of this study is to give a thorough examination of the efficacy of patent pools and other methods of collaboration for boosting innovation and access to important medications in poor nations. The specific objectives of the paper include an examination of the legal and regulatory framework governing patent pools and other collaborative mechanisms in various countries and regions, identification of the potential advantages of these mechanisms, analysis of their drawbacks and challenges, lastly, formulation of recommendations for policymakers, pharmaceutical companies, and other stakeholders on how to design and implement these mechanisms.
Keywords
Patent pool; IPR, Cooperative competition; Technology sharing; Patent infringement; India; Developing nations; Innovation; Access; Medicine; CCI; TRIPS
Introduction
Patents and the Intellectual Property (IP) system have traditionally been used to reduce risk in pharmaceutical research and development. The biopharmaceutical industry is widely regarded as one of the most risky among R & D-intensive businesses [1]. The typical medication development cost is currently projected to be $ 1.3 billion, with 15-year development duration [2]. The expense and duration of clinical stages, which are used to check a new drug’s safety, efficacy, and quality, have grown the fastest. The cost of accumulating and compiling data in a pharmaceutical registration file is approximately $ 467 million, accounting for more than 60% of the total R & D expenditure [3]. A single clinical study typically consists of over 160 operations and lasts a minimum of 780 days [1]. According to industry statistics, just 3 out of every 10 prescription pharmaceuticals generate revenue that exceeds the typical cost of research and development, and only one out of every 5,000 molecules studied becomes a pharmaceutical medicine [4]. In order to lower these risks and promote investment in bio-pharmacological R & D, patents have been vital. In fact, it is estimated that between 60% and 65% of inventions in the pharmaceutical industry would not have been developed or disclosed without patents [5].
Another pertinent problem is the significant disparity in biopharmaceutical R & D between pharmaceuticals manufactured for industrialised and developing countries. The industrialised world continues to consume the lion’s share of drugs, with 95% of new medication sales occurring in the United States, Europe, and Japan [6]. In addition, while consumption is increasing in some developing countries (By 2020, it is anticipated that the E7 nations-Brazil, China, India, Indonesia, Mexico, Russia, and Turkey-will represent around 1/5th of worldwide pharmaceutical sales), the majority of pharmaceutical R & D still takes place within the frameworks of mature and developed markets [6]. This means that illnesses that mostly afflict poor populations in developing nations, including tuberculosis and malaria, continue to necessitate the development of novel medications and treatments. This means that the poor world faces a 2nd layer of danger, in that the foregoing trend is likely to continue even while the developed world experiences more advanced treatments that allow for longer and higher quality lives.
A Global Strategy and Plan of Action for fostering innovation and improving access to healthcare products in underdeveloped countries was created by the Intergovernmental Working Group on Public Health, Innovation, and Intellectual Property (IGWG) of the World Health Organisation in response to this concern [7]. Certain non-traditional methods have been examined within the framework of this global plan to manage the hazards involved with developing pharmaceutical treatments for neglected diseases, particularly when it comes to Intellectual Property Rights (IPRs). WHO members are specifically asked to ‘examine the viability of upstream and downstream voluntary patent pools to support the development and accessibility of medical devices and health products’ [8].
Furthermore, the policy encourages members to investigate and, where appropriate, support a variety of incentive systems for research and development, such as prize awards, with the goal of tackling diseases that disproportionately afflict developing nations. This implies that one of the major reasons for the neglect of diseases that disproportionately affect poor countries is that patents do not sufficiently handle the risks in pharmaceutical R & D directed at these markets [9]. With alternate solutions like patent pools, it is proposed to restrict risk on both levels-drug developments generally and drug development for commercially unattractive areas in particular. A relatively new and much contested strategy for fostering and promoting innovation in essential medicinal items is patent pools. Patent pools are a specific structure in which members cross-license patents and other intellectual property in order to gain access to vital technology for specific products.
Patent pools have previously been used successfully in IT, consumer electronics, and other businesses with a huge volume of patents. However, they may not be as appropriate to the pharmaceutical and biotechnology businesses. The purpose of this study is to give a thorough examination of the efficacy of patent pools and other methods of collaboration for boosting innovation and access to important medications in poor nations. The specific objectives of the paper include an examination of the legal and regulatory framework governing patent pools and other collaborative mechanisms in various countries and regions, identification of the potential advantages of these mechanisms, analysis of their drawbacks and challenges, identification of best practises and lessons learned from successful and unsuccessful collaborations, lastly, formulation of recommendations for policymakers, pharmaceutical companies, and other stakeholders on how to design and implement these mechanisms.
Research Methodology
The doctrinal approach is a form of legal study that entails examining current laws, rules, and policies connected to a certain subject. In this study, the doctrinal methodology is applied to investigate the legal and regulatory framework governing patent pools and other cooperative arrangements for fostering innovation and facilitating access to critical medications in developing nations. To pinpoint best practises and takeaways, the paper will also examine case studies.
Patent pools
Strong IP rights create a ‘tragedy of the anti-commons,’ preventing knowledge from being used by anybody but the patent holder [10]. When relevant patents for a particular good or process are held by numerous different companies, the tragedy of the anti-commons is most apparent; this poses an important obstacle to access and innovation because anyone wishing to supplement or improve on existing knowledge must secure rights from each individual patent holder. Patent pools seek to avert this calamity by increasing the accessibility of patented goods.
Broadly speaking, a patent pool is an ‘agreement between 2 or more patent owners to provide one another or 3rd parties a licence to a group of those patents’ [11]. Such pools function by ‘collecting a series of patents that relate to the use of a particular technology so that they can be efficiently licensed to those making, using, or selling that technology’ [12]. Therefore, patent pools provide a wider accessibility to new technologies by making patents more accessible to those who do not have patents.
For a variety of factors, patent pools have been employed in many industries, and they have created a number of benefits for both patent holders and industries across the board. As a result, the use of patent pools to promote access to technology is not a new occurrence. Patent pools simultaneously lower the risks involved with adopting patented technologies and reduce the time and expense associated with individual licence negotiations, in addition to providing greater access to innovations.
Legal Framework: International and National
A brief description of the International legal framework: Intellectual property, TRIPS, and the Doha declaration: According to the 1994 WTO Agreement on Trade Related Aspects of Intellectual Property (TRIPS), all nations that are WTO members are required to give pharmaceutical corporations medication patents [13]. All members of TRIPS are required to abide by the minimal global standards for the protection of Intellectual Property (IP). TRIPS specifically requires that members draft their municipal patent laws in a way that grants qualified patent holders of medicines a monopoly in the market and allows them to exclude others from producing, using, selling, or importing the medication for the duration of the patent-20 years following the filing of the patent excluding the time needed to evaluate the patent [14].
By removing generic competition from the market, pharmaceutical companies with patents are able to charge extravagant prices for drugs that are out of reach for the majority of people in the developing countries, which acts as a primary impetus for price reductions of drugs [15].
Strong patent protection for pharmaceuticals is something that TRIPS mandates, yet it has drawn much international criticism. One of these criticisms is that the TRIPS ‘was the product of duress by powerful states against weak states rather than a bargain struck by sovereign equals,’ achieved through the promise of improved commercial access and the threat of trade sanctions [16]. Since wealthy countries control over 80% of the world drug patents, they profit far more from robust IP protection than poor countries, and as a result, the protections offered by TRIPS tend to be substantially more favourable to developed countries [17]. Strong worldwide IP protection is advocated as being important for pharmaceutical businesses to recover the R & D expenses related to bringing a medicine to market, which is essential for future innovation. The question of whether such robust IP protection is necessary in order to recover R & D costs has generated a lot of discussion. Due to a lack of transparency, it is occasionally unclear how much money is actually spent on R & D by pharmaceutical companies and how much is funded by national governments [18]. The amount of money spent on marketing new pharmaceuticals, which is frequently covered by R & D expenses, is also unknown; some estimates indicate that nearly 1/3rd of all sales revenue is really spent on marketing new products, which is roughly twice as much as is spent on R & D [18]. Strong IP protection proponents assert that it might also act as ‘a powerful tool for development’, in the long run; this will make new and improved medications accessible in developing nations [19].
Unfortunately, robust patent protection has served to significantly hinder access to medicine in the developing countries, where resources are scarce and the cost of higher-quality medications is still mostly out of reach [20]. The 2001 Doha declaration on the TRIPS agreement and public health spoke directly to this issue.
It acknowledged the ‘gravity of the public health problems affecting many developing and least developed countries, especially those resulting from HIV/AIDS, tuberculosis, malaria, and other epidemics’ and emphasized that the ‘TRIPS agreement does not and should not prevent Members from taking measures to protect public health’ [21].
In a nutshell the Doha declaration confirmed that nations, especially those in developing regions, have the right to use TRIPS’ flexibility provisions to advance access to vital medicines in light of public health requirements [20].
The Doha declaration was significant because it acknowledged the necessity for affordable medicine procurement methods in developing nations. But TRIPS also recognised this, albeit less overtly, by allowing for a transition period that permitted developing and Least Developed Countries (LDCs) to put off achieving full TRIPS compliance [22]. Developing and least-developed countries were able to postpone full compliance until 2005 and 2021, respectively, by establishing alternative compliance timetables for nations at varying stages of development [23]. LDCs are nevertheless exempt from extending patent protection to pharmaceutical corporations as a result of these various compliance schedules, and are given the greatest amount of freedom to create and market medications [23].
Unfortunately, a number of LDCs have already granted pharmaceutical patents despite not fully utilising the compliance extension, frequently as a result of trade pressure from rich countries.
Despite the robust patent protection mandated by TRIPS, the 20-year period of patent protection under TRIPS is not indefinite. A variety of flexibilities that poor nations can and should employ to advance access to medication are espoused in TRIPS and confirmed by the Doha declaration [24]. One such flexibility that developing countries have utilised is the power to grant Compulsory Licences (CLs), which permit a country or a 3rd party permitted by that country to utilise a patented innovation without the patent holder’s approval in exchange for payment of a governmentdetermined royalty [25]. Although contentious and infrequently utilised in the past, this significant exception to a patent holder’s claim to exclusivity has recently become more often adopted by poor countries and has had a significant impact on boosting access to medicine.
Other policies that affect access to medicine in developing nations exist in addition to TRIPS’ strict requirements for pharmaceutical patent protection. In particular, Free Trade Agreements (FTAs) between developing and developed nations call for developing nations to enact ‘TRIPS-Plus’ provisions, which require developing nations to enact national patent laws that go above and beyond TRIPS strict requirements [26]. Many FTAs, for instance, call for the adoption of data exclusivity rules, which serve to extend a company’s patent term past 20 years by preventing generic manufacturers from using earlier clinical trial data to demonstrate a bioequivalent generic’s efficacy for a while [26]. Due to the high cost of clinical trials and the additional market entry obstacles created by this legislation, the development of generic drugs is further hampered.
Owing to the high expenses of performing expensive clinical trials, which would greatly increase the costs involved in bringing a generic drug to market, many generic businesses may decide to wait out the data exclusivity period instead [27]. Additionally, certain free trade agreements limit a nation’s capacity to issue CLs. Such limitations, particularly in the field of HIV/AIDS, might negatively affect a nation’s access to medical activities. Lack of transparency in patent information is another issue. As a result of the confusion over which medications are patented and which are not, this has made the access issue in developing nations worse and made it more difficult for such nations to obtain generic medications. The 2005 avian flu outbreak provides an illustration of the issue with transparency [28]. Many developing nations started debating their choices with regard to either voluntary or obligatory licences as a result of the need for significant quantities of generic versions of the medication oseltamivir. Roche, the patent holder, later informed some nations that there were no active patents [29].
Patent pool: The Indian legal framework
Prior to 2005, India had a process patent system that was utilised by industries like biopharmaceuticals to create a strong generics business [30]. Indian companies saw little resistance from the preponderance of anticommons because they only produced bio-generics and not innovative products, for which they held many process patents [31]. However, after the Patent (Amendment) Act of 2005 a new system of product patents is anticipated to make it difficult for people to obtain patented knowledge [32]. Many industries, like biotechnology, nanotechnology, and others, have a patentable landscape that is becoming more and more fragmented, requiring numerous patents to create a single product [33]. This entails negotiating several licences and paying multiple licencing fees, which raises the cost of the finished good.
The problem of securing the various licences required for a product that is also affordable to the marginalised in developing countries can be resolved with the help of a patent pool, which offers some hope. The Indian Patent (Amendment) Act of 2005 (IPA) doesn’t contain any sections that directly deal with the collective protection of patents through a patent pool, but it also doesn’t have any provisions that may legally prevent the formation of such a pool [34]. Given that the basis of a patent pool is a number of cross-licensing agreements between patent holders and licencing agreements with 3rd parties wishing access to the patents in a pool, it will be crucial to investigate the provisions dealing with licencing and assignment under the IPA and assess the resulting consequences for patent pooling. The IPA’s Section 68 allows for the written contract-based assignment of patent rights [35].
Under Section 69 of the Act, a person must send a written request to the Controller for the registration of their title or, as appropriate, notice of their interest in the register if they acquire a patent, a share in a patent, or any other interest in a patent by an assignment, transmission, or other method [35].
The provisions of the Indian Patent Act that prevent the introduction of specific stringent conditions into a contract for the licencing of a patent are also significant in the context of patent pools. Licencing agreements linked to a pool have frequently been accused of adding restrictive requirements, which has resulted in a lot of antitrust action worldwide. Section 140 of the Indian Patent Act makes it illegal to include clauses in a licence to work any patent-protected technique, manufacture, or use a patented product [35].
In India, Competition Act of 2002, certain IPR licencing agreements are prohibited if they are regarded to be ‘anti-competitive in nature’ [36]. As per Section 3(1) of the Act, no enterprise, association of enterprises, person, or association of persons shall enter into any agreement regarding the production, supply, distribution, storage, acquisition, or control of goods or the provision of services that voids under Section (2) and has or is expected to have a significant negative impact on competition within India [35]. The section primarily discusses the horizontal and vertical forms of anti-competitive agreements. Horizontal agreements are covered by Section 3(3), which states that any agreement made between businesses, groups of businesses, individuals, or associations of individuals, as well as any practise adopted or decision made by groups of businesses, individuals, or associations of individuals, including cartels, engaged in the same or similar trade of goods or provision of services, and which
a) directly or indirectly determines purchase or sale price;
b) limits or controls production, supply, markets, technical development, investment or provision of services;
c) shares the market or the source of production or the provision of services by allocating the market’s geographical region, its product or service category, its client base, or in any other manner comparable to this;
d) directly or indirectly results in bid rigging or collusive bidding, shall be presumed to have an appreciable adverse effect on competition and, therefore, void
e) shares the market or source of production or provision of services by way of allocation of geographic market, or type of goods or services, or number of customers in the market, or in any other similar way.
To the extent that a joint venture agreement improves efficiency in the production, supply, distribution, storage, acquisition or control of commodities or the provision of services, nothing in this sub-section shall apply to that joint venture agreement.
Section 3(4) deals with vertical agreements and states that any agreement between businesses or individuals at different points in the production chain or markets regarding the production, supply, distribution, storage, price, or trade of goods or the provision of services, including
a) tie-in arrangements;
b) exclusive supply agreements;
;c) exclusive distribution agreements;
d) refusal to deal; and
e) resale price maintenance, shall be an agreement in contract [20].
Section 3(5) of the Competition Act has a specific clause stating that any reasonable restrictions necessary to protect intellectual property while exercising those rights do not constitute anti-competitive agreements. The Act makes no mention of or definition for the phrase ‘reasonable conditions [20].’ The Competition Commission of India (2002) published an advocacy booklet that interprets this to mean that Section 3 will apply to any IPR with unreasonable requirements attached [20]. According to this document, licencing agreements will be subject to competition law if they are not expected to have a detrimental effect on pricing, quantities, quality, or types of goods and services, as well as if they are not substantially in contradiction to the package of rights that come with IPRs. The article continues with examples of licencing agreements that may be anti-competitive in nature, including ones that divide markets among companies that would have competed using different technologies, exclusive licencing agreements, including cross-licensing by parties with combined market power, agreements that effectively combine the R & D efforts of 2 or a small number of businesses that may properly engage in R & D in the relevant field, and patent grants back.
The Competition Commission of India (CCI), in this document, names a group of actions that have a restrictive or anti-competitive character [37]. It defines patent pooling as a ‘restrictive practice, which will not constitute being a part of the bundle of rights forming part of an IPR’. The circumstances under which the patent pool turns anticompetitive are specified. The CCI claims that this occurs when businesses in a manufacturing sector decide to pool their patents, agree not to provide licences to other parties, and set quotas and pricing at the same time. They might enjoy above-average profits and deter new competitors from entering the industry. It will be particularly challenging for outside competitors to compete if all the technology is concentrated in a small number of hands due to a pooling arrangement. It also lists a number of other practises as ‘anti-competitive,’ including tie-in agreements, price fixing, prohibiting a licensee from using competing technology, agreements to pay royalties even after a patent has expired or for unpatented know-how, the insertion of a requirement not to contest the validity of the IPR in question, the requirement that any acquired know-how or IPR be returned to the licensor and not to grant licences to anyone else, package licencing, and more. The CCI’s illustrative examples will probably act as a reference for decisions in circumstances where licencing practises, including crosslicensing, may be deemed to be ‘anti-competitive.’ In order to prevent the pool from becoming the target of antitrust lawsuit, these could offer useful suggestions for those in charge of organising patent pools in India. According to case law from developed nations, patent pools have been charged with being anti-competitive in situations where multiple of the CCI-cited restrictive practises have been found to exist [38].
Health and patent pool: The global scenario
The use of patent pools in global health programmes to expand access to ARVs and promote ND R & D represents an innovative way to go beyond the status quo [39]. The goal of patent pools for global health, like other patent pools, is to address some of the issues with the present market-based IP landscape [40]. Global health patent pools have the ability to reduce costs, promote needs-driven research, and promote innovation by managing IP from a public health standpoint, making medication more readily and affordably available. While learning from other patent pools’ experiences can be helpful, patent pools for global health are unique [40]. Patent users that use the IP in the pool without also donating to it make up a distinct group of patent donors who licence their technology into the pool for non-profit purposes. In contrast to the majority of patent pools, which either feature industry-wide patent pools that are prompted by the need for an industry-wide standard, or cross-licensing among competitors to exchange patents necessary for the manufacture of a particular technology. This alters the dynamics of patent pools for global health in comparison to other patent pools because they are required not by financial or practical considerations, but rather by humanitarian considerations, and as a result, the right incentives must be present to persuade patent holders to contribute to the pool. The Medicines Patent Pool (MPP) and WIPO research Consortium are 2 new patent pools for global health [41]. Although they are both patent pools for global health, the inputs and focus on diseases are very different. The MPP is a patent pool for ARVs that tries to get around IP restrictions that prevent the development of more modern, less harmful, and more effective ARVs. The patents used to create and produce patented ARVs are the pool’s inputs. The pool intends to boost generic ARV production and eventually encourage the creation of new FDCs that are more suited to the needs of developing nations. Research serves as a patent pool for substances, information, and expertise linked to NDs [42].
The goal of research is to promote upstream creation of completely new medications for NDs, as opposed to the MPP’s focus on downstream innovation. Research aims to accelerate the development of new ND drugs and lessen the financial and administrative burdens associated with licencing arrangements, which may be required after product development, by enabling information exchange and giving users access to patents, data, and know-how related to NDs [43]. In order to create ND medications and technologies, people need to be able to find and collaborate with the owners of the necessary patents, data, and knowhow. This is where the actual value of research lies because it makes partnerships possible, removes informational barriers, and lowers transaction costs. Additionally, it might assist in resolving the tragedy of the anti-commons in relation to upstream expertise, goods, and methods that are now underutilised because various parties have varying claims to the materials required for producing downstream goods. By doing this, it solves the patent thicket issue that now inhibits scientists from exchanging knowledge and applying it to create new drugs, vaccines, diagnostics, and other technologies for NDs.
Does intellectual property impede innovation?
Tragically, lack of access to available medical treatments is thought to be the cause of 1/3rd of disease-related fatalities globally [44]. HIV/AIDS shows a particularly serious public health concern, with 33.3 million people expected to have the disease by the end of 2023 [45]. To meet this goal, 95% of HIV-positive individuals (PLHIV) must receive an HIV diagnosis, 95% of patients must receive Antiretroviral Therapy (ART) that lasts a lifetime, and 95% of PLHIV must receive viral suppression for the improvement of their health and the prevention of HIV transmission to others [45]. HIV/AIDS medications are essential for both saving patients’ lives and stopping the disease’s spread. Access for a large number of patients in underdeveloped nations depends on the drug’s pricing as well as its physical accessibility at a distributor [46].
Additionally, because individuals become resistant to antiretroviral medications, newer medications will be required, many of which have the advantage of having fewer adverse effects. The foundation for determining whether public health is safeguarded and meaningful access to medicines is noted by the World Health Organization’s (WHO) Commission on Intellectual Property Rights, Innovation, and Public Health (CIPIH). The framework takes into account accessibility, acceptability, effectiveness, affordability, and whether the treatment is ‘of the lowest possible cost’ in order to ensure access.
Despite the fact that this right has been recognised as a fundamental human right and that ‘to gradually achieve complete realisation, access to medications is a crucial component,’ there are still barriers in place that prevent millions of people, especially in the developing world, from receiving treatment. Access to these treatments for patients is usually hampered by monopolies and expensive prices brought on by intellectual property rights. Patents also give owners of the rights the ability to combine their original ideas with new ones that come later, such as fixed dose combinations or better ways to store or transport medicines. The high intellectual property barriers can create what has been called the ‘tragedy of the anti-commons [10].’ The continuing development of beneficial new products might be hampered by patent thickets, which result from circumstances of fragmented ownership or the blocking of crucial ‘upstream’ research is patented. The end result is that ‘No one has an effective right to utilise anything, and several owners have the right to keep others out of a limited supply.’ The process of converting rights into useful private property after an anti-common has emerged is frequently violent and slow. The intellectual property issues that prohibit or impede adequate access for persons in the developing world must be addressed in order to advance the right to health. Patent pools, which function inside the current intellectual property framework, are one method for addressing the issues with access and creativity.
Obstacles to anti-retroviral
IP restrictions continue to be a substantial barrier to accessing ARVs in developing countries despite the abundance of obstacles to pharmaceutical access in those nations [47]. The patents on many older ARVs have expired, making them more affordable, while newer, less toxic ARVs are still protected by patents, making them mostly unaffordable. Without generic competition, which is a primary driver of price reductions, medicine prices would continue to be so high as to be unaffordable for many people. This is why patent protection remains such a big barrier [48].
The introduction of generic pharmaceuticals to the market is significant since it not only makes bioequivalent, less expensive medications available, but also drives down the cost of brand-name medications. Another major obstacle to the development of FDCs is patent law. A generic business must negotiate individual licencing arrangements with several patent holders in order to manufacture an FDC that contains more than one patented component. This process can be expensive and time-consuming [49]. Additionally, even if the other components are readily available, patent owners can simply decline to licence their inventions, preventing the creation of an FDC. Because of this, some essential 1st and 2nd line FDCs are either unavailable or insufficiently supplied in developing countries.
The treatment of HIV/AIDS in developing countries requires paediatric and heat stabilised formulations, which are not necessary in wealthy countries, despite the fact that a number of ARVs are accessible there. There is no market-driven method of manufacturing these formulations. Paediatric and heat stabilised formulations are needs that are unique to the developing world, much like NDs, therefore pharmaceutical corporations have little incentive to conduct R & D and innovate in these fields.
Less than 7% of all HIV patients who get treatment belong to the limited paediatric ARV market [50]. Paediatric HIV is actually an ND due to the limited size of the market, and there is a serious market failure with regard to paediatric formulations. There isn’t a large market for paediatric ARVs in affluent countries because so few kids are born with HIV. As a result, pharmaceutical companies have little incentive to create paediatric formulations. Because paediatric HIV treatment varies as children progress through various developmental phases, the already tiny paediatric ARV market is further divided into more specialised niche markets. As children transition from infancy to maturity, this necessitates not just the development of one paediatric formulation but also the development of numerous medicines in various doses and delivery modalities [51]. As a result, only one triple FDC is appropriate for kids, and only 1/3rd of ARVs are currently accessible in paediatric formulations [52]. The availability of any ARVs for children is pretty surprising, and this is probably because of the financial commitments made by groups like the Clinton HIV/AIDS Initiative, which has encouraged the creation of paediatric formulations by providing pharmaceutical companies with a market.
India’s contribution to developing countries access to antiretroviral
India frequently referred to as ‘the pharmacy of the world’ for its role in providing affordable medications to underdeveloped nations [53]. More than 80% of the yearly ARV purchase volumes for poor nations are produced by Indian manufacturers, including the 88% of all FDC formulas and the 91% of all formulas for children [54].
India established itself to play this role by fully utilising TRIPS’s latitude, refusing to acknowledge pharmaceutical patents until TRIPS compelled such patent protection in 2005 [37]. India was able to establish a robust generic pharma industry as a result of this action by the Indian government because there were no patent restrictions on creating affordable medications for the developing world. Since the beginning of India’s generic medication sector, high volume, low margin items like generic ARVs have been the main emphasis of Indian companies’ business models.
Since TRIPS was put into effect in 2005, there have been changes in the environment surrounding inexpensive drug access. Even though the bulk of 1st line ARVs are off patent, newer 2nd and 3rd line treatments that are more effective, have less side effects, and lead to less drug resistance have been copyrighted, preventing Indian generic enterprises from delivering the pharmaceuticals for Developing Countries [55]. Etavirine, raltegravir, and maraviroc are examples of drugs that are needed for the treatment of patients who have failed first-line therapy but are not yet generic because they are presently patented in India [56]. India’s ability to offer developing nations affordable drugs has been significantly limited because generic manufacturers are unable to enter the market and generate the kind of competition that leads to the kind of enormous price reductions seen in the past with first line ARVs [57].
Despite being required to grant patent protection, India has maintained its commitment to public health by maintaining a high threshold for patentability, reserving patent protection for only original drugs, and refusing patent protection for minor improvements to existing drugs or reformulations of well-known chemical compounds.
This high patentability criterion has led to the denial of patents for a number of significant ARVs, including TDF, darunavir, LPV/r, and atazanavir [53]. This has made it possible for Indian generic businesses to keep offering developing nations inexpensive ARVs. The notion of India as the ‘pharmacy of the developing world’ is ebbing [58]. Negotiations for a free trade agreement could endanger India’s capacity to provide developing nations with affordable medications. However, given that it is one of the few developing nations with sizable manufacturing capacities, India will continue to be crucial in providing medicines to developing countries.
India and other emerging economies in neglected drugs R & D
Recently, emerging economies have contributed to ND R & D [59]. A Brazilian manufacturer has created a treatment for skin infections in leprosy patients in the recent years, while an Indian generic company has created a medication for the treatment of visceral leishmaniasis. Brazil has committed 236.31 million on a variety of projects as part of a pilot R & D programme, in addition to drug research, with the ultimate goal of discovering therapies for Chagas disease [60].
Countries with developed generic pharmaceutical businesses, such as China, India, Brazil, and South Africa, are interested in participating in ND R & D since, unlike multinational pharmaceutical corporations situated in developed nations; their people are affected by NDs [61]. They are also in a stronger position to engage in ND R & D since, despite the modest market for ND medications, these countries’ R & D expenses are far cheaper than those of developed nations [62]. This is due to a variety of factors, including lower fixed asset costs (i.e., lower costs of constructing manufacturing facilities), cheaper labour, lower costs of regulation, efficient manufacturing processes, a large suitable population that can be easily and inexpensively recruited for clinical trials, and low-cost marketing.
Discussion
Case study: The medicine patent pool’s first license: The U.S. National Institute of Health
When the White House stated that the National Institutes of Health (NIH) had granted a licence for NIH-owned patents on darunavir on September 30, 2010, the MPP acquired its first licence. This gift from the NIH ‘builds on the President’s prior commitment to support humanitarian licencing policies to ensure that medicines developed with U.S. taxpayer money are available off patent in developing countries,’ according to the statement. This licence, which related to the NIH’s method of treatment patents on the significant protease inhibitor darunavir, was non-exclusive and free of royalties [63].
The World Bank’s definition of low and middle-income nations served as the license’s broad geographic reach [64]. This license’s scope of use was broadened to include ‘treatment and prevention of medical conditions affecting humans.’ This licence, the first for the MPP, was praised by a large portion of the public health community. The MPP’s objectives are perfectly aligned with the clauses that make the licence non-exclusive, royalty-free, and applicable to all low and middle-income nations.
Additionally, the new institution had political support when the US government agreed to licence its patents to the MPP. The MPP was given credibility by NIH, the greatest supporter of biological research in the world, and might serve as a model for other publically financed research organisations, academic institutions, or patent owners. Although this first licence from the NIH was undoubtedly a step in the right direction, it is crucial to remember that these licences did not allow the production of generic darunavir for the benefit of HIV/AIDS patients.
Other patents pertaining to darunavir are held by Tibotec/ Johnson and Johnson in addition to the NIH, a private pharmaceutical firm. Unfortunately, despite the fact that 3 of its medications-darunavir (DRV), etravine (ETR), and rilpivirine-are on the MPP’s list of target medications, Tibotec/Johnson and Johnson has not yet entered in negotiations with the MPP [18]. Sub-licensees may not produce the drug for use or sale in countries where the darunavir patents are in effect until Johnson and Johnson engages in formal discussions with the MPP and licences its products to the pool. Johnson and Johnson officially refused to engage in negotiations on December 19, 2011, despite efforts to persuade the corporation to participate in the MPP. By granting licences to the patent pool, pharmaceutical companies should demonstrate the CSR they claim to practise and guarantee that people in developing nations have access to life-saving medications.
Generally speaking, the research results indicate that the focus placed on patent pools and other comparable processes by international organisations, such as the WHO, for the aim of stimulating the development of new pharmaceutical treatments for neglected diseases may be, at least in part, incorrect.
The results of this study indicated that while many projects and programmes are sponsored overall by the initiative outlined here, very few are actually engaged in R & D activities intended to generate new pharmaceutical medications. In fact, a sizable percentage of the projects’ efforts are concentrated on bringing generic versions of already-approved treatments to poor nations rather than on R & D for brand-new medications. In other words, rather than achieving their claimed goal of fostering the creation of novel medications for unmet medical needs, patent pools and other non-proprietary models attempt to introduce less expensive substitutes for the current medications.
These strategies appear to have been successfully applied by several intermediary projects, such as the TB Alliance and the Malaria Vaccine projects, to deliver a number of goods to emerging markets. In other words, approaches that have a direct link between IPR holder’s compensation mechanisms and particular pharmaceuticals or technology may be more effective than patent pools (Figure 1).
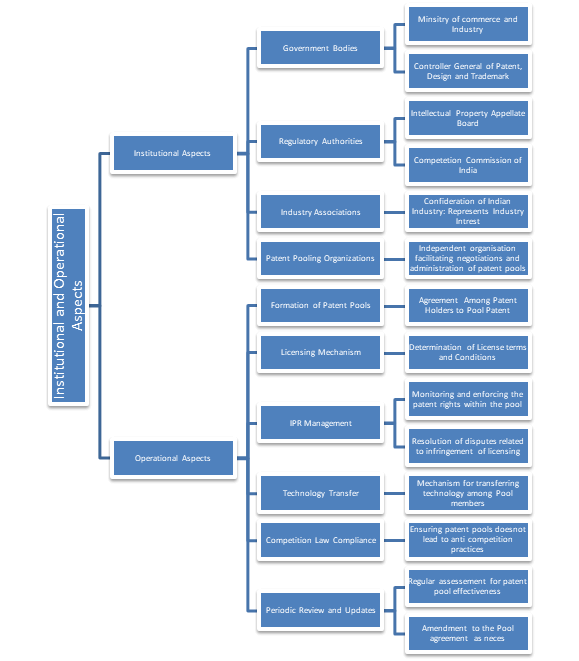
Figure 1: Showing the institutional and operational aspects of the patent pools in India
Conclusion and Suggestions
In underdeveloped nations, where many people lack the finances to buy life-saving treatments, access to crucial medicines is a critical concern. This issue may be solved by encouraging innovation and lowering access barriers through patent pools and other cooperative arrangements. However, the extent of collaboration, participation incentives, and the regulatory framework all has an impact on how effective they are. The researcher has covered the possible advantages of patent pools and other cooperative methods in this paper, as well as their drawbacks and difficulties. The ability of patent pools to promote the exchange of information, assets, and technology across various stakeholders is one of its main benefits. These systems can help lower transaction costs and legal obstacles to accessing crucial medicines by pooling patents and licencing agreements. For developing nations, where intellectual property rights can be a significant barrier to acquiring affordable medications, this can be very helpful. Nevertheless, the extent of collaboration and the incentives for involvement determine how effective patent pools are. For instance, if critical patents are left out or if certain populations’ demands are not met, patent pools might not be effective. Therefore, it is crucial to create patent pools and other forms of cooperation that are specifically adapted to the requirements of developing nations and engage a wide range of stakeholders.
Governments may play a key role in promoting these collaborations by offering rewards for participation, ensuring accountability and openness, and establishing supportive regulatory frameworks. Governments may, for instance, provide grants or tax incentives to businesses that take part in patent pools or other cooperative methods. They can also create legal frameworks that support cooperation and expand access to necessary medications. In addition to patent pools and other forms of cooperation, it’s critical to address more general problems with developing nations’ access to medication. These consist of healthcare facilities, pricing guidelines, and intellectual property rights. For instance, governments can implement regulations that support the production and distribution of generic medications. They can also bargain with pharmaceutical firms to lower drug costs for developing nations. The problem of access to necessary medications cannot be solved by patent pools and other cooperative arrangements, it is crucial to understand this. These strategies can only be successful if they are a part of a larger plan that deals with systemic access constraints. As a result, it is crucial to establish a holistic approach that incorporates cooperation between many stakeholders and sectors. In conclusion, patent pools and other cooperative arrangements may foster innovation and provide access to critical medications in underdeveloped nations. Their success, however, depends on careful planning, execution, and larger initiatives to overcome structural impediments to access to medications. The development and implementation of successful initiatives for expanding access to vital medications in developing nations requires collaboration between governments, pharmaceutical corporations, and other stakeholders. Only then will we be able to guarantee that everyone has access to the necessary life-saving treatments.
Acknowledgement
None.
Conflict Of Interest
Authors have no conflict of interest to declare.
References
- M.P. Pugatch, Patent pools and collaborative initiatives: Assessing the efficacy of alternatives to IP in the development of new pharmaceutical drugs, especially for neglected diseases: An empirical analysis, Eur J Risk Regul, (2011):566-571.
- O.J. Wouters, M. McKee, J. Luyten, Estimated research and development investment needed to bring a new medicine to market 2009-2018, JAMA, 323(2020):844-853.
- J.A. DiMasi, R.W. Hansen, H.G. Grabowski, The price of innovation: New estimates of drug development costs, J Health Econ, 22(2003):151-185.
- D.W. Light, R. Warburton, Demythologizing the high costs of pharmaceutical research, BioSocieties, 6(2011):34-50.
- F.M. Scherer, The pharmaceutical industry: Handbook of health economics, 1(2000):1297-1336.
- S.A. Laird, S. Laird, M. Burningham, The botanical medicine industry: In the commercial use of biodiversity, Routledge, (2019):78-116.
- T.M. Jones, WHO intergovernmental working group on public health, innovation and intellectual property, Lancet, 376(2010):21.
- E. Burrone, Patent pooling in public health: The Cambridge handbook on public-private partnerships, intellectual property governance, and sustainable development, (2018).
- P. Trouiller, P. Olliaro, E. Torreele, J. Orbinski, R. Laing, et al. Drug development for neglected diseases: A deficient market and a public-health policy failure, Lancet, 359(2002):2188-2194.
- S. Kampari, Tragedy of digital anti-commons, in S-38.042 seminar on networking business, Helsinki University of Technology Networking Laboratory, 2004.
- A. Eisenberg, Understanding patent pools for global health: Assessing their value in promoting access to essential medicines, Duke University, 2014.
- E.R. Gold, T. Piper, J.F. Morin, L.K. Durell, J. Carbone, et al. Preliminary legal review of proposed medicines patent pool, Innovation Partnership, (2007).
- E. Hoen, TRIPS, pharmaceutical patents, and access to essential medicines: A long way from Seattle to Doha, Chi J Int'L L, 3(2002):27.
- J. Watal, Pharmaceutical patents, prices and welfare losses: Policy options for India under the WTO TRIPS agreement, World Economy, 23(2000):733-752.
- D.A. Balto, Pharmaceutical patent settlements: The antitrust risks, Food Drug Law J, 55(2000):321.
- P. Drahos, J. Braithwaite, Intellectual property, corporate strategy, globalisation: TRIPS in context, Wis Int LJ, 20(2001):451.
- R.H. Wade, What strategies are viable for developing countries today? The World Trade Organization and the shrinking of 'development space', Rev Int Polit Eco, (2003):621-644.
- B. Shaw, J. Mestre-Ferrandiz, Talkin' about a resolution: Issues in the push for greater transparency of medicine prices, Pharmacoeconomics, 38(2020):125-134.
- R. Hartungi, Could developing countries take the benefit of globalisation? Int J Soc Eco, 2006.
- D. Matthews, WTO decision on implementation of paragraph 6 of the DOHA declaration on the TRIPs agreement and public health: A solution to the access to essential medicines problem? J Int Eco Law, 7(2004):73-107.
- European commission, 2023.
- WHO, Implications of the doha declaration on the TRIPS agreement and public health, 2002.
- M Banda, WTO trips agreement: A hindrance to the economic development of least developed countries? The case of malawi and rwanda, WIPO-WTO, 125(2019).
- J. Sundaram, India's trade-related aspects of intellectual property rights compliant pharmaceutical patent laws: What lessons for India and other developing countries? Info Comm Tech L, 23(2014):1-30.
- R.C. Bird, Developing nations and the compulsory license: Maximizing access to essential medicines while minimizing investment side effects, J Law Med Ethics, 37(2009):209-221.
- S. Frankel, Challenging TRIPS-plus agreements: The potential utility of non-violation disputes, J Int Eco Law, 12(2009):1023-1065.
- D.P. Goldman, D.N. Lakdawalla, J.D. Malkin, J. Romley, T. Philipson, The benefits from giving makers of conventional 'small molecule' drugs longer exclusivity over clinical trial data, Health Aff, 30(2011):84-90.
- E.R. Sedyaningsih, S. Isfandari, T. Soendoro, S.F. Supari, Towards mutual trust, transparency and equity in virus sharing mechanism: The avian influenza case of Indonesia, Ann Acad Med Singap, 37(2008):482.
- J.P. Love, Recent examples of the use of compulsory licenses on patents, Knowledge Eco Int, 8(2007).
- T. Papaioannou, A. Watkins, J. Mugwagwa, D. Kale, To lobby or to partner? Investigating the shifting political strategies of biopharmaceutical industry associations in innovation systems of South Africa and India, World Dev, 78(2016):66-79.
- S.E. Reid, S.V. Ramani, The harnessing of biotechnology in India: Which roads to travel? Technol Forecast Soc Change, 79(2012):648-664.
- The Patent Act, 1970.
- K. Singh, L.S. Gangwar, B. Prakash, A.S. Yadav, Patent pool: An effective intellectual property competition, The changing landscape for the libraries and librarians: Issues and challenges, 252(2022).
- S. Mani, India's patenting record since TRIPS compliance of her patent regime, AJTI, 29(2021):427-454.
- Indian patent act, 1970.
- The competition act, 2002.
- Competition commission of India, 2023.
- Y. Pai, N. Daryanani, Patents and competition law in India: CCI's reductionist approach in evaluating competitive harm, J Antitrust Enforc, 5(2017):299-327.
- S.J. Hoffman, K. So, P. Galappati, S. Rans, A. Tsang, et al. Assessing 15 proposals for promoting innovation and access to medicines globally, Ann Glob Health, 80(2014):432-443.
- J. Bermudez, E. Hoen, The UNITAID patent pool initiative: Bringing patents together for the common good, Open AIDS J, 4(2010):37.
- A. Nilsson, Making norms to tackle global challenges: The role of Intergovernmental organisations, Res Policy, 46(2017):171-181.
- S. Morris, A. Smith-Chaigneau, Interactive TV standards: A guide to MHP, OCAP, and JavaTV CRC press, 2012.
- S.K. Cohen, N.V. Munshi, Innovation search dynamics in new domains: An exploratory study of academic founders' search for funding in the biotechnology industry, Technol Forecast Soc Change, 120(2017):130-143.
- K. Cox, The medicines patent pool: Promoting access and innovation for life-saving medicines through voluntary licenses, Hastings Sci Tech LJ, 4(2012):291.
- World health organisation, 2023.
- S.R. Schwartz, J. Bassett, I. Sanne, R. Phofa, N. Yende, et al. Implementation of a safer conception service for HIV-affected couples in South Africa, AIDS, 28(2014):277.
- J.C. Cohen-Kohler, L. Forman, N. Lipkus, Addressing legal and political barriers to global pharmaceutical access: Options for remedying the impact of the agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) and the imposition of TRIPS-plus standards, Health Econ Policy Law, 3(2008):229-256.
- N. Davey, Overcoming patent barriers to increase access to medicines: A new path forward for compulsory licensing, Harv JL Tech, 35(2022).
- K. Satyanarayana, S. Srivastava, Patent pooling for promoting access to Antiretroviral Drugs (ARVs): A strategic option for India, Open AIDS J, 4(2010):41-53.
- S.S. Forsythe, W. McGreevey, A. Whiteside, M. Shah, J. Cohen, et al. Twenty years of antiretroviral therapy for people living with HIV: Global costs, health achievements, economic benefits, Health Aff, 38(2019):1163-1172.
- H.K. Batchelor, J.F. Marriott, Formulations for children: Problems and solutions, Br J Clin Pharmacol, 79(2015):405-418.
- World Health Organization, Access to antiretroviral drugs in low-and middle-income countries: Technical report July 2014, 2014.
- T. Bazzle, Pharmacy of the developing world: Reconciling intellectual property rights in India with the right to health: TRIPS, India's patent system and essential medicines, Geo J Int'l L, 42(2010):785.
- C.A. Passarelli, The role of intellectual property rights in treatment access: Challenges and solutions, Curr Opin HIV AIDS, 8(2013):70-74.
- F. Pascual, Intellectual property rights, market competition and access to affordable antiretrovirals, Antivir Ther, 19(2014):57-67.
- O. Ebrahim, A.H. Mazanderani, Recent developments in HIV treatment and their dissemination in poor countries, Infect Dis Rep, 5(2013).
- F.M. Abbott, J.H. Reichman, The doha round's public health legacy: Strategies for the production and diffusion of patented medicines under the amended TRIPS provisions, J Int Econ Law, 10(2007):921-987.
- J.K. Plahe, D. McArthur, After TRIPS: Can India remain 'the pharmacy of the developing world'? South Asia: J South Asia Stud, 44(2021):1167-1185.
- International monetary fund, 2023.
- L.S. Sangenito, M.H. Branquinha, A.L.S. Santos, Funding for chagas disease: A 10-year (2009-2018) survey, Trop Med Infect Dis, 5(2020):88.
- R. Rezaie, A.M. McGahan, S.E. Frew, A.S. Daar, P.A. Singer, Emergence of biopharmaceutical innovators in China, India, Brazil, and South Africa as global competitors and collaborators, Health Res Policy Syst, 10(2012):1-13.
- Z. Ezziane, Essential drugs production in Brazil, Russia, India, China and South Africa (BRICS): Opportunities and challenges, Int J Health Policy Manag, 3(2014):365.
- S. Juneja, A. Gupta, S. Moon, S. Resch, Projected savings through public health voluntary licences of HIV drugs negotiated by the Medicines Patent Pool (MPP), PLOS One, 12(2017).
- T. Bright, S. Wallace, H. Kuper, A systematic review of access to rehabilitation for people with disabilities in low-and middle-income countries, Int J Environ Res Public Health, 15(2018):2165.
Copyright: © 2024 Mohd Kaif, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

