Research Article: Journal of Drug and Alcohol Research (2023) Volume 0, Issue 0
Role of Habb-e-Jawahar in Attenuating Nicotine Withdrawal in Rats
Raka Jain*Raka Jain, Department of Psychiatry, National Drug Dependence Treatment Center, AIIMS, New Delhi, India, Email: rakajain2009@gmail.com
Received: 02-Jan-2023, Manuscript No. JDAR-22-55512; Editor assigned: 04-Jan-2023, Pre QC No. JDAR-22-55512 (PQ); Reviewed: 18-Jan-2023, QC No. JDAR-22-55512; Revised: 23-Jan-2023, Manuscript No. JDAR-22-55512 (R); Published: 30-Jan-2023, DOI: 10.4303/JDAR/236220
Abstract
Background: The Habb-e-Jawahar (HJ) is one of the most important drugs used in the indigenous system of Unani medicine in India. It is considered to be one of the potent drugs of the nervous system and provides strengthening to the vital organs.
Purpose: To evaluate the efficacy of Unani drug, HJ to attenuate mecamylamine precipitated nicotine withdrawal in nicotine-dependent rats.
Methods: Male adult Wistar albino rats (n=54) were used in the study and were made physically dependent by subcutaneous infusion of nicotine (9.0 mg/kg/day) via a 7-day osmotic pump, while the control rats received saline. The three different doses of HJ (15.9 mg/kg, 22.5 mg/kg and 38.4 mg/kg) were given orally for 7 days. Nicotine withdrawals were precipitated with nicotine antagonist, mecamylamine (1 mg/kg), 2 hours after the test dose on day 7. Somatic signs of withdrawal were scored for 15 minutes using Gellert–Holtzman (GH) rating scale followed by a measurement of motor activity. The drug, Bupropion was used as a positive control. Serum levels of nicotine (cotinine) and corticosterone were estimated by ELISA.
Keywords
Habb-e-Jawahar; Nicotine; Withdrawal; Bupropion; Mecamylamine
Introduction
Tobacco is one of the world’s most serious public health threats. Every year, it kills almost 8 million people around the world [1]. Of the world’s 1.3 billion tobacco users, low and middle-income nations account for more than 80%. Tobacco smoking is a complex behavior involving both psychological and pharmacological components. Nicotine is the major alkaloid found in tobacco and is responsible for its tolerance and dependence. Repeated use of nicotine produces physical dependence and tolerance in humans and rats [2]. Its effect on mood and cognition are strong supplements for smokers that contribute to their dependence.
Nicotine is a weak base, lipophilic in nature, and easily absorbed by the membrane. In the brain, nicotine binds to the nicotine acetylcholine receptor (nAChR). Neuronal nAChR are ligand-gated ion channels with high permeability to Ca++, and the receptor consists of five subunits [3,4]. There are nine α subunits (α2-α10) and three β subunits (β2-β4) in the mammalian brain. The most abundant receptors are α4β2, α3β4, and α7, with α7 being homomeric. The main mediator of nicotine dependence is α4β2 receptors. In mice, disrupting the β2 subunit genes reduces nicotine’s behavioral effects, whereas reinserting the gene into the ventral tegmental region restores nicotine’s behavioral effect [5,6].
Repeated exposure to nicotine increases the number of binding sites on the nAChR causes desensitization of receptors which plays an important role in the development of tolerance and dependence [2]. The desensitized α4β2 nAChR becomes responsive during abstinence and nicotine binding of these receptors during smoking reduces craving and withdrawal [3]. In mice, chronic nicotine administration or administration of nAChR antagonist induces affective, somatic, and cognitive signs of withdrawal [7]. During withdrawal when challenged with nAChR antagonist mecamylamine, higher levels of corticosterone and corticotropin-releasing factor (CRF) are detected in the central nucleus of the amygdala [8]. This suggests that the Hypothalamic-pituitary-adrenal (HPA) axis plays a vital role in the processing of the negative affective states associated with nicotine withdrawal [9].
Presently, one of the classical drug used in the clinical setups is Bupropion, an antidepressant used to treat major depression, craving, and other withdrawal effects by inhibiting dopamine reuptake. But there are also some common side effects of Bupropion like headache, vomiting, nausea, dizziness, troubled sleeping, etc. There are numerous therapies that are used in smoking cessation but due to limited effects or side-effects, indicate the need for alternate medicine like herbal medicines. Plants and natural products are a potential source of novel and safer compounds to use for drug withdrawal management [10,11].
The classical Unani formulation, Habb-e-Jawahar (HJ) is a Unani pharmacopeial drug [12]. It is recommended as a nervine and cardiac tonic drug and prescribed for neurological and cardiac disorders [13]. It also maintains body temperature, removes chronic debility [14], provides strengthening to the vitals, regaining vitality during convalescence [13,15-19]. HJ contains 22 indigenous ingredient drugs of animal, plant, and mineral sources, as shown in Table 1. The ingredient drugs and quantity are mentioned according to the National Formulary of Unani Medicine, part-V (Table 1).
| S. No. | Drug | Scientific name | Quantity (g) | Action |
|---|---|---|---|---|
| 1 | Abresham Muqarraz | Silk cocoon | 10 | Memory tonic, Cardio-tonic, Exhilarant, Demulcent (Anonymous, 2009) |
| 2 | Busad Ahmar | Red coral | 10 | Cardio-tonic, Haemostatic, Siccatic (Khan, 2009; Ghani, 2011) |
| 3 | Jaiphal | Myristica fragrans | 5 | Cardiotonic, Stomachic, digestive (Anonymous, 2009, Zaheer et al. 2016) |
| 4 | Jadwar | Delphinium denudatum | 5 | Nervine tonic, Antidote to poison, Anti-inflammatory, Anti pyretic (Anonymous, 2009) |
| 5 | Dana Ilaichi Khurd | Elettaria cardamomum | 10 | Cardiotonic, Stomachic, Carminative (Anonymous, 2009) |
| 6 | Daroonaj Aqrabi | Doronicum hookeri | 10 | Nervine stimulant, Stomachic (Ghani, 2011) |
| 7 | Zahar Mohra | Serpentin stone | 10 | Antidote to poison, Vital organ tonic, Purifier, Exhilarant (Ghani, 2011) |
| 9 | Aqeeq Surkh | Agate | 10 | Cardiac tonic, Aphrodisiac (Ghani, 2011) |
| 10 | Ood Gharqi | Aquilaria agollocha | 5 | Nervine tonic, Anti septic, Expectorant, appetizer (Anonymous, 2009) |
| 11 | Firoza | Tarquize | 6 | Cardiotonic, Nervine tonic, Exhilerant (Ghani,2011) |
| 12 | Kahruba | Vateria indica | 5 | Cardiotonic, Hepato-protective, Haemostatic (Ghani,2011) |
| 13 | Lajward | Lapis lazuli | 5 | Nervine stimulant, Cardiotonic, Haemostatic (Ghani,2011) |
| 14 | Marwareed | Pearl | 5 | Exhilarant, Enhances body faculties and vital force, Anti-depressant (Ghani,2011) |
| 15 | Narjeel Daryaee | Lodoicea maldivica | 8 | Antidote to poison, removes waste and toxic humours (Ghani,2011) |
| 16 | Yaqoot Surkh | Ruby | 16 | Antidote to poison, protect innate energy of body, exhilarant (Ghani,2011) |
| 17 | Yashab Sabz | Green Jade | 12 | Cardio and nervine tonic, stomachic (Ghani,2011) |
| 18 | Zafran | Crocus sativus | 3.5 | Cardio tonic, Anti-inflammatory, Anti septic (Anonymous, 2009) |
| 19 | Ambar | Ambegris | 2 | Antidote to poison, Protects the vital organs, (Ghani,2011) |
| 20 | Warq Nuqra | Silver leaves | 20 | Stimulant, General body tonic Aphrodisiac, Protects vital faculty (Ghani, 2011) |
| 21 | Arq Gulab | Rose water | 300ml | Cardiotonic, Anti- inflammatory, Frigorific (Anonymous, 2009) |
| 22 | Warq Tila | Gold leaves | QS | Anti-depressant, Exhilarant, Cardio-nervine tonic (Ghani, 2011) |
Table 1: Active Ingredients of Habb-e-Jawahar and their action.
Some drugs have an antidotal effect against poisons and are used for withdrawal symptoms having a synergistic therapeutic activity. One of the ingredient drugs, Jadwar (Delphinium denudatum) is reported to have a significant effect in attenuation of morphine dependence. The Unani literature also supports this, citing its beneficial effects on nervous disorders and opium addiction. Another ingredient drug, Jaiphal or Nutmeg (Myristica fragrans) has been reported to significantly reduce morphine withdrawal syndrome [20-25].
These findings suggest that different ingredients of HJ are useful in heart, liver, and neurological disorders. Also, have a significant effect in decreasing morphine withdrawal. The whole HJ formulation has not been scientifically evaluated for its therapeutic effects. Therefore, taking into consideration the therapeutic effect of HJ and its individual ingredients, HJ was selected for the current study to evaluate its effects on mecamylamine precipitated nicotine withdrawal in nicotine-dependent rats.
Material and Methods
Drugs
For the study, Unani formulation drug HJ was procured from Hamdard laboratories, New Delhi, India (Batch No. OKO0001, Mfg. Lic.No. U-212/78/2014). The pills were powdered with the help of mortar (ceramic bowl) and pestle (Blunt ceramic object) by crushing and grinding the drug until the desired texture is achieved. Ethanol (99.9%) and distilled water were taken in equal amounts for making Hydro-alcoholic (1:1) extract. The freshly prepared extract was dissolved in normal saline and given orally with the help of an oro-gastric cannula. Nicotine tartrate dihydrate salt and Bupropion hydrochloride were procured from Tokyo Chemical Industry (TCI), Japan. Mecamylamine was procured from Cayman Chemical Company, USA and Ketamine hydrochloride of Claris Injectable Limited, India. Nicotine and mecamylamine were administered subcutaneously while ketamine (50 mg/Kg) was given intraperitoneally. Drug mecamylamine was given at the volume of 1 mg/kg and Bupropion at 20 mg/kg body weight expressed with the free base.
Preparation of Habb-e-Jawahar (HJ)
Huboob (Pills) are small, round, and uniformly shaped medicinal preparations, neither very hard nor very soft. HJ was prepared according to the National for Formulary of Unani Medicine (NFUM-V).
Animals
Adult male Wistar Albino rats (n=48) weighing 175 g-250 g were individually housed in plastic cages at a temperature (23 ± 1°C) with a 12 h. light/dark cycle. Food and water were provided ad libitum in the home cages. Animals were acclimatized to the laboratory condition for 5 days prior to the experiment. The All India Institute of Medical Sciences, Institutional Animal Ethics Committee approved the experimental protocol (Reference No. 973/IAEC/16).
Induction of physical dependence
Under ketamine anesthesia, all rats were implanted subcutaneously (SC) with Alzet 2ML1 (7 days) mini osmotic pump (Alza Scientific Products, Palo, Alto, CA, USA) in the scapular region filled with Nicotine tartrate di-hydrate in normal saline. The nicotine concentration was adjusted based on the body weight of animals to deliver continuous infusion at the rate of 9 mg/kg/day (equivalent to 3.15/kg/ day expressed as the nicotine base) for 7 days. Each pump was primed for 4 h in normal saline, prior to implantation [26-28].
Experimental design
The current study was carried out to assess the effect of hydro-alcoholic extract of HJ on mecamylamine-induced withdrawal in nicotine-dependent rats. A total of 54 rats were used in the study. They were randomly assigned to the 9 groups containing 6 rats each. The control rats (n=6) were implanted with Alzet mini-osmotic pumps filled with saline and experimental rats (n=6) were implanted with nicotine-filled Alzet Mini-osmotic pumps followed by mecamylamine challenge on day 7 (1 mg/kg; i.p.). Three groups of rats (n=6, per group) were implanted with nicotine- filled Alzet Mini-osmotic pumps and were treated with three different doses of HJ (15.9 mg/kg, 22.5 mg/kg and 38.4 mg/kg) followed by mecamylamine challenge to assess their effect on nicotine withdrawal. Three treatment control groups of rats (n=6, per group) were implanted with Alzet mini-osmotic pumps filled with saline and were treated with three different doses of HJ (15.9 mg/kg, 22.5 mg/kg, 38.4 mg/kg) followed by mecamylamine challenge. One positive study control group was also run (n=6, per group) in which rats were subcutaneously implanted with mini-osmotic pumps filled with nicotine and saline and were treated with an atypical anti-depressant, Bupropion (20 mg/kg; i.p.) followed by mecamylamine challenge on day 7 days to assess its effect on nicotine withdrawal and to compare it with rats treated with test drugs, HJ (Table 2).
| Days | 15.9 mg/kg HJ (n=12) | 22.5 mg/kg HJ (n=12) | 38.4 mg/kg HJ (n=12) | 20 mg/kg, Bupropion (n=12) |
|---|---|---|---|---|
| 3 days of acclimatization | ||||
| Day 0 (Behavioural) | (No drug given) | (No drug given) | (No drug given) | (No drug given) |
| Day 1 | Nic+HJ15.9 | Nic+HJ22.5 | Nic+HJ38.4 | Nic+Bup |
| Day 2 (Behavioural) | Nic+HJ15.9 | Nic+HJ22.5 | Nic+HJ38.4 | Nic+Bup |
| Day 3 | Nic+HJ15.9 | Nic+HJ22.5 | Nic+HJ38.4 | Nic+Bup |
| Day 4 (Behavioural) | Nic+HJ15.9 | Nic+HJ22.5 | Nic+HJ38.4 | Nic+Bup |
| Day 5 | Nic+HJ15.9 | Nic+HJ22.5 | Nic+HJ38.4 | Nic+Bup |
| Day 6 (Behavioural) | Nic+HJ15.9 | Nic+HJ22.5 | Nic+HJ38.4 | Nic+Bup |
| Day 7 (Behavioural) | Nic+HJ15.9+Mec | Nic+HJ22.5+Mec | Nic+HJ38.4+Mec | Nic+Bup+Mec |
Note: Nic=Nicotine, Sal=Saline Bup= Bupropion (20mg/kg, i.p.), Mec=Mecamylamine (1mg/kg s.c.), HJ=Habb-e-Jawahar (15.9, 22.5, 38.4 mg/kg body wt.), mg=Milli gram, Kg=Killogram, s.c.=Subcutaneous, i.p.=Intraperitoneal. Control rats were administered with saline in place of nicotine.
Table 2: Mode of drug administration.
Behavioral observation
Assessment of motor activity: The motor activity was used to measure by Activity Monitor (True Scan Photo beam Sensor-E63-22, Coulbourn Instruments, Inc. USA). On days 0, 2, 4, 6, and 7, before the mecamylamine challenge, the activity was monitored for 25 minutes at 5 minutes intervals. The animals were weighed on the same days prior to measurements of motor activity on each day and 2 hours following the mecamylamine challenge.
Measurement of Gellert-Holtzman’s rating score (GHscore): Rats were weighed on day 7 of nicotine infusion (168 hours after the pump was implanted). They were given mecamylamine challenge (1 mg/kg) subcutaneously after 2 hours of test drug (HJ) to precipitate withdrawal [27,28]. After the mecamylamine challenge, animals were immediately placed into an activity cage and visually observed for 15 minutes. They remained in the activity cage for an additional 10 minutes.
The somatic signs of withdrawal were assessed by using the global Gellert–Holtzman (GH) rating scale [28-30]. The individual somatic signs assessed were derived from a global GH rating scale that included graded signs of weight loss, abdominal constrictions, number of escape attempts, and checked signs (simply scored as present or absent) such as facial fasciculation/teeth chattering, diarrhea, swallowing movements, chromodacryorrhea, ptosis, profuse salivation, penile grooming/erection/ejaculation, abnormal posture, and irritability upon handling. With the exception of weight loss, graded signs were assigned a weighting factor from 1 to 4 based on the frequency of appearance, on the global GH rating scale. The checked signs were given values of 2–7 depending upon the withdrawal signs observed, but regardless of the frequency of appearance. All the somatic signs were recorded for the first 15 minutes after the mecamylamine injection, with the exception of weight loss. The weight loss was calculated as the difference between the weight measured immediately prior to mecamylamine injection and the second determination made 60 minutes after the injection. No food was made available to the rats during this period. Thus, for the first 15 minutes, the score of precipitated withdrawal was observed along with the measurement of gross activities [27].
Measurement of serum cotinine and corticosterone levels
Rats were anesthetized by ketamine injection. Thereafter, 1 ml of blood was drawn from the heart, and plasma was separated and stored at -80ºC for serum corticosterone and nicotine (cotinine) levels (Koob et. al., 2007). Plasma corticosterone (EC3001-1, Assaypro, USA) and cotinine (nicotine metabolite) (BC-ER141403, Biocodon Technologies, USA) levels were estimated by using ELISA as per manufacturer’s guidelines. Samples or, standards were run in duplicate and the ELISA plate was read by Multimode ELISA Reader (M200 PRO, TECAN, and Austria). Finally, according to the optical density values of the standard and samples, serum cotinine and corticosterone levels (ng/ml) were calculated by the statistical software.
Data analysis
All analysis was performed by using the software SPSS 22. For comparing motor activity within a group repeated measure ANOVA (Two-way ANOVA) followed by multiple comparisons using the Bonferroni test was applied. One-way ANOVA with Bonferroni test for multiple comparisons was used for comparing motor activity between the groups. A Chi-square test was applied to analyze the individual behavioral signs. The p<0.05% was considered significant.
Results
Comparison of motor activity
Repeated measure ANOVA with Bonferroni (post-hoc) test revealed significant increase in motor activity with nicotine exposure in the nicotine administered rats with respect to saline treated rats from day 0 to 4 [(F4, 20)=3.223, p<0.01]. As shown in Figure 1, non-significant decrease in motor activity was observed on day 6. Further, significant increase in motor activity was observed in nicotine dependent rats after mecamylamine challenged on day 7 (p<0.001) whereas, no change was observed in saline treated rats after mecamylamine challenge. On the other hand, no significant change was observed in the motor activity of HJ [15.9 mg/kg: (F4, 20)=2.002, p>0.05; 22.5 mg/kg: (F4, 20)=1.775, p>0.05; 38.4 mg/kg: (F4, 20)=1.874, p>0.05] and Bupropion [(F4, 20)=1.698, p>0.05] treated rats as compared to nicotine treated ratsover the period of 7 days. No significant change was observed in HJ (at all three doses, p>0.05) and Bupropion (p>0.05) treated rats after mecamylamine challenged with respect to day 6 time point. One-way ANOVA with post-hoc test revealed that there was signigicant difference in motor activity between the nicotine and the HJ treated rats [15 mg/kg: (F4, 25)=20.96, p<0.001; 22.5 mg/kg: (F4, 25)=21.615, p<0.001; 38.4 mg/kg: (F4, 25)=23.292, p<0.001] at all three doses on day 7 after mecamylamine challenge. Hence, all doses of HJ (15.9 mg/kg, 22.5 mg/kg and 38.4 mg/kg) appeared to be effective in attenuating the hyperlocomotion in nicotine treated rats (Figure 1).
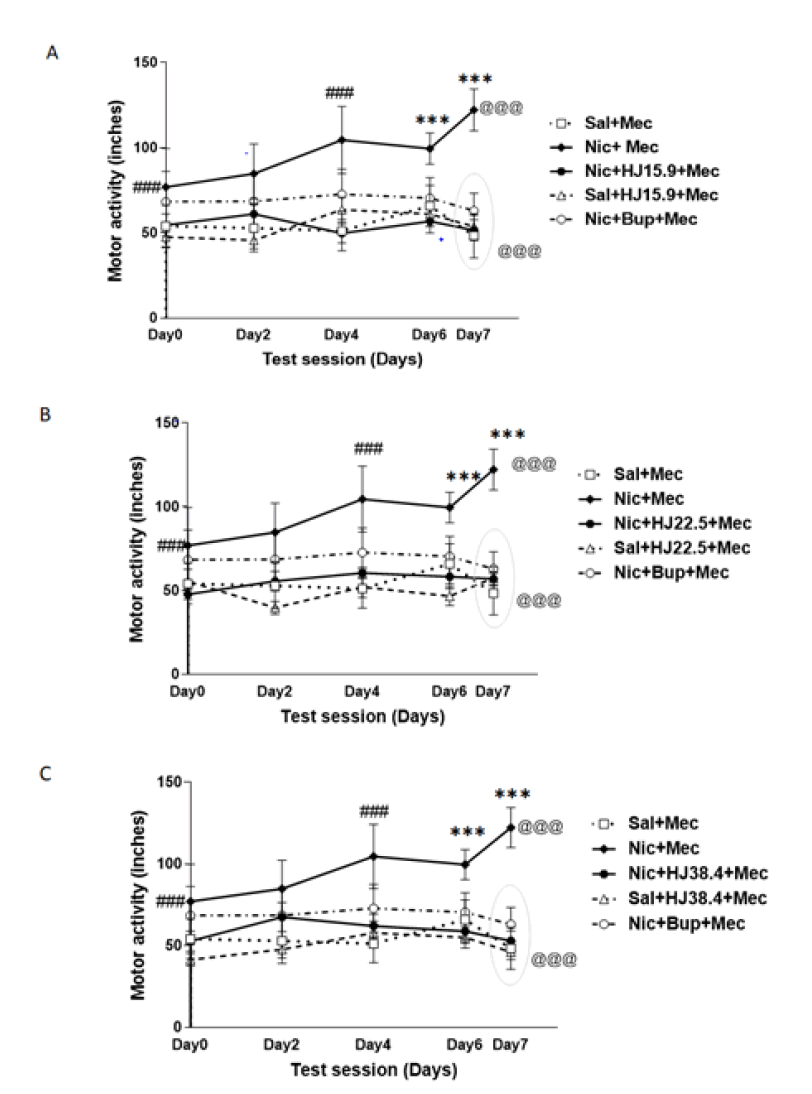
Figure 1: Motor activity of nic, sal and HJ: A) 15.9 mg/kg; B) 22.5 mg/kg; C) 38.4 mg/kg treated rats. Motor activity is expressed as average distance traveled (inches) after nic, sal, and HJ administration over a test session of 7 days. Each point represents the mean central distance and SEM. of 6 rats. ### indicates a significant increase (p<0.001) in motor activity over a period of 4 days with nicotine exposure and *** indicates a further significant increase (p<0.001) in motor activity after mecamylamine challenge in nic+mec- treated rats, based on repeated measure ANOVA within groups. @@@ indicates a significant difference (p<0.001) in motor activity after the mecamylamine challenge on day 7 between the nic+mec, sal+mec and HJ treated groups by One-way ANOVA.
Comparison of Gellert-Holtzman’s (GH) rating score
When comparing mecamylamine precipitated nicotine treated rats to saline and Bupropion treated rats, a oneway ANOVA with post-hoc test demonstrated a significant increase in GH-Score during withdrawal in mecamylamine precipitated nicotine treated rats. There was a significant reduction in the GH-score of HJ treated rats than in the mecamylamine precipitated nicotine treated rats [F (8,45)=5.437, p<0.001]. The GH scores and individual behavioral signs in saline, nicotine, and HJ-treated rats are shown in Table 2. Therefore, oral administration of HJ was found to be effective in attenuating the mecamylamine precipitated withdrawal in nicotine-dependent rats at all test doses (15.9 mg/kg, 22.5 mg/kg and 38.4 mg/kg) as shown in Figure 2.
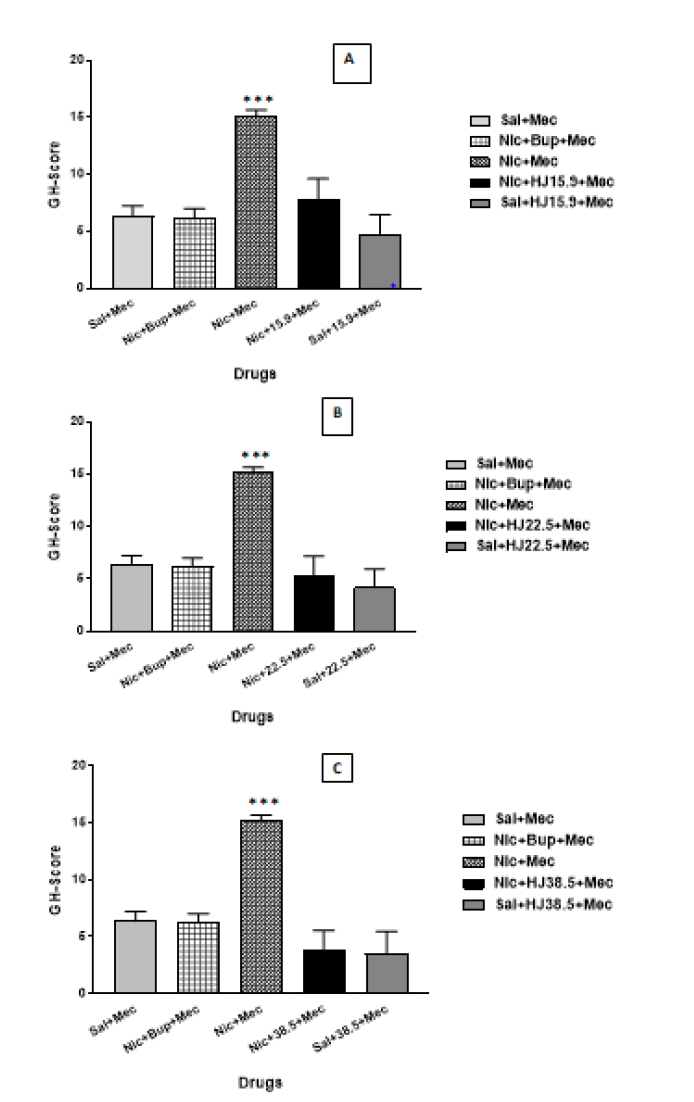
Figure 2: GH-score of sal, nic, and HJ (A: 15.9 mg/kg; B: 22.5 mg/kg; C: 38.4 mg/kg) treated rats. Each bar represents the mean cAMP and S.E.M. of 6 rats. *** indicates significantly higher levels of GH-score (p<0.001) in nic+mec treated rats after mecamylamine challenge compared with sal, nic+Bup and HJ treated rats by One-way ANOVA between the groups.
Graded signs: The graded and checked signs were analyzed using the Chi-square test. The escape attempts were significantly higher in mecamylamine precipitated nicotine treated rats compared with saline and Bupropion. At all test doses of HJ given prior to mecamylamine challenge displayed complete suppression of escape attempts (15.9 mg/kg: χ=9.387, p<0.001; 22.5 mg/kg: χ=10.572, p<0.001; 38.4 mg/kg: χ=12.846, p<0.001) (Table 3).
| Behavioural signs | Nic. | Nic+Bup | HJ (15.9 mg/kg) | HJ (22.5 mg/kg) | HJ (38.4 mg/kg) | |
|---|---|---|---|---|---|---|
| GH-Score | 15.166*** | 6.66*** | 7.83*** | 5.33*** | 3.83** | |
| Graded Signs | ||||||
| No. of Esc Attempts | ||||||
| Two-Four | 0 (0%) | 3 (50%) *** | 2 (33.3%) *** | 2 (33.3%) *** | 1 (16.6%) *** | |
| Five–Nine | 2 (33.3%)* | 1 (16.6%) *** | 1 (16.6%) *** | 1 (16.6%) *** | 0 (0%) *** | |
| Ten or more | 4 (66.6%)* | 0 (0%) *** | 0 (0%) *** | 0 (0%) *** | 0 (0%) *** | |
| Checked Signs | ||||||
| Facial Fasciculation | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 5 (83.3%) | |
| Ptosis | 6 (100%) *** | 2 (33.3%) *** | 1 (16.6%) *** | 1 (16.6%) *** | 0 (0%) *** | |
| Abnormal Posture | 6 (100%) *** | 3 (50%) ** | 3 (50%) ** | 3 (50%) ** | 0 (0%) *** | |
| Ejaculation | 6 (100%) *** | 2 (33.3%) *** | 4 (66.6%) | 4 (66.6%) | 2 (33.3%) *** | |
| Irritability | 6 (100%) *** | 4 (66.6%) | 3 (50%) ** | 3 (50%) ** | 2 (33.3%) *** | |
Note: a. The minimum criterion for statistical significance was when p value was less than 0.05 (*p<0.05, **p<0.01, ***p<0.001). Values are mean of six rats in each group (n=6). b. Data represent the percentage of subjects in each group that showed individual signs during a 15-minute test. Abbreviations: Nic=Nicotine, Sal=Saline, Bup=Bupropion, HJ=Habb-e-Jawahar (15.9, 22.5, 38.4 mg/kg body wt.), mg=Milligram, Kg=Killogram.
Table 3: Behavioural signs of rats at different doses of HJ in nicotine treated rats.
Checked signs: Abnormal posture, ptosis, ejaculation and irritability were highly prominent in mecamylamine challenged nicotine treated rats than in saline and bupropion treated rats. The oral administration of HJ significantly reduced the abnormal posture (15.9 mg/kg: χ=7.577, p<0.01; 22.5 mg/kg: χ=8.196, p<0.01; 38.4 mg/kg: χ=12.196, p<0.001), ptosis (15.9 mg/kg: χ=11.177, p<0.001; 22.5 mg/ kg: χ=12.164, p<0.001; 38.4 mg/kg: χ=13.64, p<0.001) and irritability (15.9 mg/kg: χ=6.577, p<0.01; 22.5 mg/ kg: χ=7.61, p<0.01; 38.4 mg/kg: χ=10.26, p<0.001). The 38.4 mg/kg dose of HJ significantly suppressed the ejaculation (χ=10.26, p<0.001), whereas, lower doses: 15.9 mg/ kg and 22.5 mg/kg decreased the rate of ejaculation but not to the significant level. Fascial fasciculation was equally observed in all nicotine, saline and Bupropion treated rats. The 38.4 mg/kg dose of HJ showed slight decrease in facial fasciculation but was found to be non-significant. No change was observed with oral administration of 15.9 mg/ kg and 22.5 mg/kg HJ (Table 3).
Comparison of Serum corticosterone levels
Figure 3 shows the serum corticosterone levels of mecamylamine precipitated withdrawal in nicotine, saline, bupropion, and HJ treated rats. A one-way ANOVA demonstrated an increase in serum corticosterone levels during withdrawal in mecamylamine-precipitated nicotine-dependent rats. The post-hoc test confirmed the significant increase in serum corticosterone levels in mecamylamine precipitated nicotine treated rats compared with saline and Bupropion treated rats. The rats treated with three different oral doses of HJ (15.9 mg/kg, 22.5 mg/kg and 38.4 mg/ kg) significantly suppressed the elevated corticosterone levels in mecamylamine-challenged nicotine treated rats [F (8,45)=24.112, p<0.001] (Figure 3).
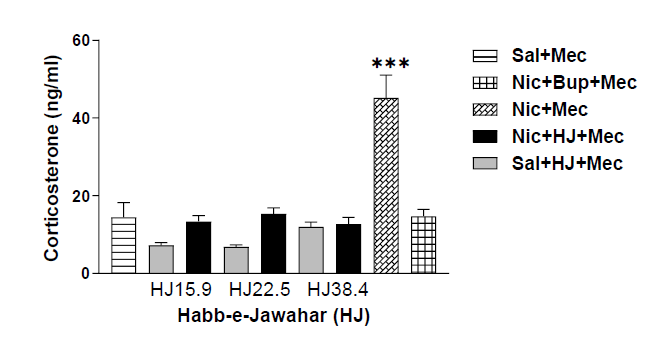
Figure 3: Corticosterone levels of sal, nic, and HJ (A: 15.9 mg/kg; B: 22.5 mg/kg; C: 38.4 mg/kg) treated rats. Each bar represents the mean cAMP and S.E.M. of 6 rats. *** indicates significantly higher levels of corticosterone (p<0.001) in nic+mec treated rats after mecamylamine challenge compared with sal, nic+mec and HJ treated rats by One-way ANOVA between the groups.
Comparison of serum cotinine levels
Figure 4 illustrates the cotinine levels in saline, nicotine, bupropion, and HJ-treated rats. To assess the difference in serum cotinine levels and compare the cotinine levels across the groups, a one-way ANOVA with post-hoc test was used. The nicotine-treated rats showed significantly high cotinine levels as compared with saline-treated rats [F (8,45)=36.542, p<0.001]. There was no significant difference in nicotine levels in rats treated with the control drug, Bupropion, and test drugs of HJ (15.9 mg/kg, 22.5 mg/kg and 38.4 mg/kg) when compared to nicotine treated rats (Figure 4). Therefore, treatment drugs (Bupropion and all doses of HJ) do not show any effect on nicotine levels.
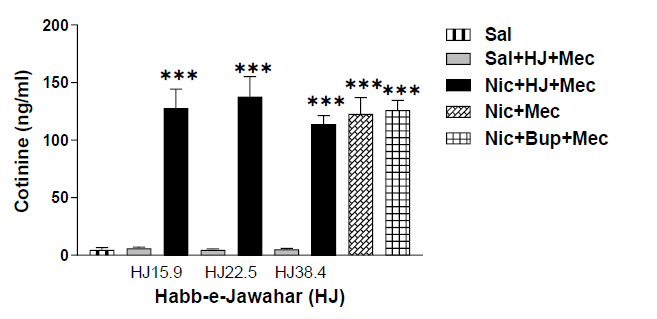
Figure 4: Cotinine levels of sal, nic, and HJ (A: 15.9 mg/kg; B: 22.5 mg/kg; C: 38.4 mg/kg) treated rats. Each bar represents the mean cAMP and S.E.M. of 6 rats. *** indicates significantly higher levels of cotinine (p<0.001) in nicotine-treated rats compared with saline-treated rats by One-way ANOVA between the groups.
Discussion
The findings of the study suggest that oral administration of the Unani compound drug, HJ attenuates the development of nicotine dependence in mecamylamine precipitated nicotine-dependent rats. To our knowledge, this is the first study of its kind to look into the effects of HJ on nicotine- dependent rats.
The open-field test was used to assess the nicotine-induced motor activity by measuring the average distance traveled. Motor activity was increased with the nicotine exposure from day 0 to day 4. On day 6 slight decrease in motor activity was observed, whereas, on day 7 further significant increase in motor activity (p<0.001) was observed when nicotine-dependent rats were challenged with mecamylamine; however, no significant change was observed in bupropion and saline-treated rats (Figure 1). The previous study has shown the effect of bupropion on various nicotine actions [31]. Our results are also in line with the findings of previous studies, showing an increase in motor activity with continuous nicotine exposure [32,33]. These results could be due to changes in the motor effects of nicotine receptor-agonism during the development of behavioral sensitization, as they are thought to play a key role in the development of addiction [34]. Thus they could be a suitable target for assessing therapies for treating addiction.
Earlier studies have shown that administration of nicotine chronically leads to physical dependence and withdrawal symptoms when challenged with antagonist [26,27,35]. The global Gellert–Holtzman’s (GH) rating scale was used in the study to measure the overall index of withdrawal intensity [28,29]. In the present study, nicotine-dependent rats showed significantly higher withdrawal intensity than the control rats when challenged with mecamylamine than in control rats (Figure 2). The graded signs such as escape attempts and checked signs, viz., ptosis, abnormal posture, ejaculation or genital grooming, and irritability were highly prominent in nicotine-dependent rats, whereas, facial fasciculation or teeth chattering were equally observed in all rats (Table 3). In contrast to this, no withdrawal sign was observed in saline-treated rats after the mecamylamine challenge. The other behavioral signs like abdominal constriction, diarrhea, weight loss, grasps, tremors, and chews were not seen. Earlier studies have shown that nicotine withdrawal also induces stress by activating the HPA-axis which causes a significant increase in plasma levels of corticosterone and ACTH (Adrenocorticotropic hormone) [36-38]. Our results are also consistent with previously published reports which indicate significantly higher levels of corticosterone in rats (p<0.001) (Figure 3). The rats administered with nicotine showed higher levels of nicotine as compared to rats treated with saline; however, bupropion treatment does not show any effect on nicotine levels in nicotine-treated rats (Figure 4).
In the current study, oral administration of HJ at three distinct test dosages (15.9 mg/kg, 22.5 mg/kg and 38.4 mg/kg) was also examined for its ability to attenuate nicotine withdrawal. The GH-score was significantly attenuated after oral administration HJ at all three doses (Figure 2). Moreover, at all doses of HJ, the checked sign-like escape attempts were suppressed and all the test doses were found to be equipotent (Table 3). The graded signs: Ptosis, ejaculation, abnormal posture, and irritability were also attenuated when treated with oral doses of HJ over a period of 7 days (Table 3). No effect was observed on facial fasciculation or teeth chattering after HJ treatment at all doses. Earlier studies have shown that the Unani drugs have been effective in reducing withdrawal signs in morphine-dependent rats [22- 25]. No change was observed over the period of 7 days in motor activity of rats treated with HJ at doses (15.9 mg/kg, 22.5 mg/kg 38.4 mg/kg) and saline (Figure 1). Therefore, all three doses of HJ were found to be effective in attenuating the hyper-locomotion observed in mecamylamine-challenged nicotine-dependent rats (p<0.001) (Figure 1).
The effect of HJ on corticosterone and cotinine levels in mecamylamine precipitated nicotine-dependent rats was also examined. The corticosterone levels were significantly decreased in mecamylamine-challenged nicotine-dependent rats after oral administration of HJ at all doses (p<0.001) (Figure 3). Our studies are also in line with the findings of the previous study suggested that the Unani compound drug Khamira Gaozaban Ambari Jadwar Ood Salib wala significantly reduced the serum corticosterone levels in rats [39]. No significant change was observed in cotinine levels after oral administration of HJ even at higher doses (Figure 4). The present study highlighted that Unani formulation HJ was significant in attenuating nicotine dependence in rats.
Conclusion
To summarize, the hydro-alcoholic root extract of Unani formulation HJ was found effective in ameliorating symptoms of nicotine withdrawal and also suppressed the increased motor activity and corticosterone levels. However, the mechanism is still unknown. Further studies are needed to understand its mechanism of action in nicotine addiction. The findings indicate that Unani formulation HJ used in traditional system of medicine have therapeutic potential and can be used as an alternative remedy in nicotine addiction and tobacco smoking cessation.
Acknowledgement
The author is thankful to Mr. Jiwan Singh Negi and Mr. Ranjeet Kumar and for technical assistance.
Conflict of Interest
None.
References
- Tobacco-World Health Organization (WHO), 2021.
- H. Wang, X. Sun, Desensitized nicotinic receptors in brain, Brain Res Rev, 48(2005):420–437.
- J.A. Dani, M. De Biasi, Cellular mechanisms of nicotine addiction, Pharmacol Biochem Behav, 70(2001):439–446.
- S. Jones, S. Sudweeks, J.L. Yakel, Nicotinic receptors in the brain: Correlating physiology with function, Trends Neurosci, 22(1999):555–561.
- U. Maskos, B.E. Molles, S. Pons, M. Besson, B.P. Guiard, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors, Nature, 436(2005):103–107.
- Y.S. Mineur, M.R. Picciotto, Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction, Biochem Pharmacol, 75(2008):323–333.
- I. McLaughlin, J.A. Dani, M.D. Baisi, Nicotine withdrawal, Curr Top Behav Neurosci, 24(2015):99-123.
- G. Koob, M.J. Kreek, Stress dysregulation of drug reward pathways and the transition to drug dependence, Am J Psychiatry, 164(2007):1149-1159.
- G.F. Koob, N.D. Volkow, Neurocircuitry of addiction, Neuropsychopharmacology, 35(2010):217–38.
- J. Ward, C. Rosenbaum, C. Hernon, C.R. McCurdy, E.W. Boyer, Herbal medicines for the management of opioid addiction: Safe and effective alternatives to conventional pharmacotherapy? CNS Drugs, 25(2011):999–1007.
- S.M. Tabatabai, S. Dashti, F. Doost, H. Hosseinzadeh, Phytotherapy of opioid dependence and withdrawal syndrome: A review, Phytother Res, 28(2014):811–830.
- The Unani Pharmacopoeia of India, Pharmacopoeia commission for Indian medicine & homoeopathy, Ghaziabad, (2009):10-11, CCRUM, Dept of AYUSH, Min of Health and Family Welfare, New Delhi, India.
- M.A. Khan, Qarabadeen-e-Azam, CCRUM, Department of AYUSH, Ministry of health and family welfare, India, (2009):79.
- S.K. Sharma, Alternate Therapies, Diamond Pocket Books Pvt. Ltd. India, (2000):113.
- M. Kabirudin, Bayaz-e-Kabir, Idara Faisal Publication, India, 1938:30-31.
- M. Abdullah, Kunz-ul-Murakkabat, Idara Matbuaat Sulaimani, Pakistan, 1987:187-188
- G. Jilani, Makhzan-ul-Murakkbat, Aijaz Publishing House, India, 1995:63.
- National formulary of Unani medicine, Department of AYUSH, Ministry of health and family welfare, 2008:10-11.
- M.H. Imam, Q. Khan, M.I. Alam, A. Goswami, M.W. Ahmad, Perceptions of maternal health and well-being in Unani system of medicine, Int J Med Health Res, 6(2020):64-67.
- S.M. Husain, Qarabadeen-e-Kabir, Urdu Translation by Hadi Husain Khan, Matba Munshi Nawal kishore, India, (1897):561–565.
- S.M. Husain, Makhzan-ul-Advia, Urdu Translation by Molvi Noor Karim, Matba Munshi Nawal Kishore, India, (1875):370–73.
- S. Zafar, M.A. Ahmad, T.A. Siddiqui, Protective role of Delphinium denudatum (Jadwar) against morphine-induced tolerance and dependence in mice, J Ethnopharmacol, 78(2001):95-98.
[Crossref ] [Google Scholar]
- S. Zafar, M.A. Ahmad, T.A. Siddiqui, Effect of roots aqueous extract of Delphinium denudatum on morphine-induced tolerance in mice, Fitoterepia, 73(2002):553-556.
- S. Rahman, R.A. Khan, A. Kumar, Experimental study of the morphine de-addiction properties of Delphinium denudatum Wall, BMC Complement Altern Med, 2(2002):6.
- I. Zaheer, S. Rahman, R.A. Khan, M. Parveen, An experimental study of ethanolic extract of Myristica fragrans in morphine dependence, Bang J Med Sci, 15(2016):224-229.
- D.H. Malin, J.R. Lake, P. Newlin-Maultsby, L.K. Roberts, J.G. Lanier, et al. Rodent model of nicotine abstinence syndrome, Pharmacol Biochem Behav, 43(1992):779–784.
- D.H. Malin, J.R. Lake, V.A. Carter, J.S. Cunningham, K.M. Hebert, et al. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat, Psychopharmacology (Berl), 115(1994):180–184.
- R. Jain, K. Mukherjee, D. Mohan, Effects of nitric oxide synthase inhibitors in attenuating nicotine withdrawal in rats, Pharmacol Biochem Behav, 88(2008):473–480.
- V.F. Gellert, S.G. Holtzman, Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions, J Pharmacol Exp Ther, 205(1978):536–546.
- R. Raghav, R. Jain, A. Dhawan, T.S. Roy, P. Kumar, Chronic co-administration of nalbuphine attenuates the development of opioid dependence, Pharmacol Biochem Behav, 175(2018): 130-138.
- J.E. Slemmer, B.R. Martin, M.I. Damaj, Bupropion is a nicotinic antagonist, J Pharmacol Exp Ther, 205(2000):321-327.
- C. Ksir, R.L. Hakan, K.J. Kellar, Chronic nicotine and locomotor activity: Influences of exposure dose and test dose, Psychopharmacology (Berl), 92(1987):25–29.
- J.R. DiFranza, R.J. Wellman, Sensitization to nicotine: How the animal literature might inform future human research, Nicotine Tob Res, 9(2007):09–20.
- H. Kayir, G. Goktalay, M. Yildirim, T.I. Uzbay, Clozapine inhibits development and expression of nicotine-induced locomotor sensitization in rats, Synapse, 63(2009):15–21.
- D.H. Malin, J.R. Lake, V.A. Carter, J.S. Cunningham, O.B. Wilson, et al. Naloxone precipitates nicotine abstinence syndrome in the rat, Psychopharmacology (Berl), 112(1993):339–342.
- M.E. Rhodes, J.S. Kennell, E.E. Belz, R.K. Czambel, R.T. Rubin, Rat estrous cycle influences the sexual diergism of HPA axis stimulation by nicotine, Brain Res Bull, 64(2004):205–213.
- J. Semba, M. Wakuta, J. Maeda, T. Suhara, Nicotine withdrawal induces sub sensitivity of the hypothalamic-pituitary-adrenal axis to stress in rats: Implications for precipitation of depression during smoking cessation, Psychoneuroendocrinology, 29(2004):215–226.
- K. Lutfy, M.C. Brown, N. Nerio, O. Aimiuwu, B. Tran, et al. Repeated stress alters the ability of nicotine to activate the hypothalamic-pituitary-adrenal axis, Neurochem, 99(2006):1321–1327.
- H. Ishaq, Anxiolytic effect of herbal medicine, khamira gaozaban ambari jadwar ood salib wala (kgj) in experimental rat models, Pak J Pharm Sci, 27(2014):289-294.
Copyright: © 2023 Raka Jain. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

